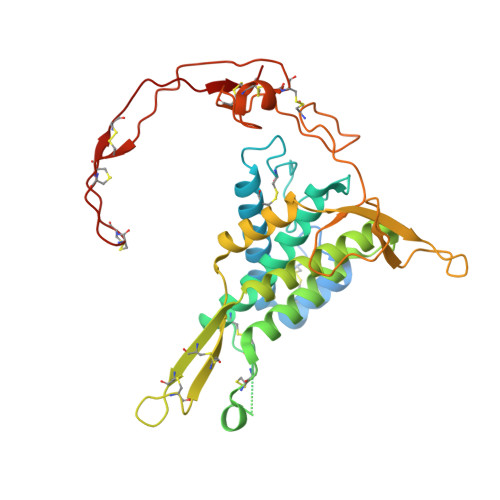
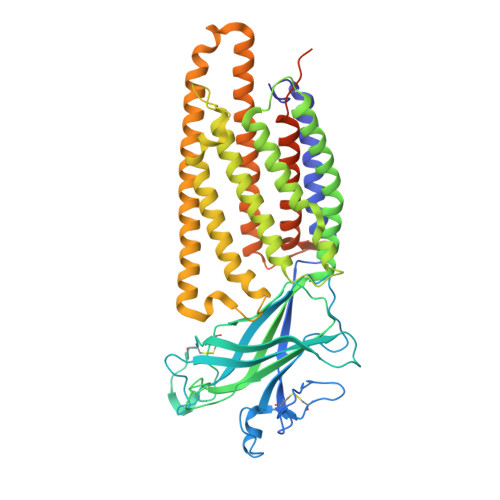
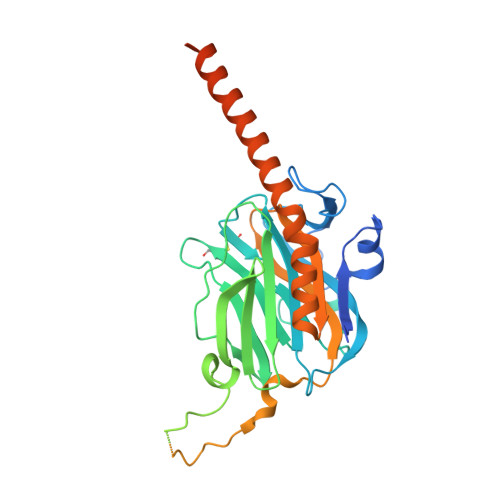
Molecular basis of Wnt biogenesis, secretion, and Wnt7-specific signaling.
Qi, X., Hu, Q., Elghobashi-Meinhardt, N., Long, T., Chen, H., Li, X.(2023) Cell 186: 5028-5040.e14
- PubMed: 37852257
- DOI: https://doi.org/10.1016/j.cell.2023.09.021
- Primary Citation of Related Structures:
8TZO, 8TZP, 8TZS - PubMed Abstract:
Wnt proteins are enzymatically lipidated by Porcupine (PORCN) in the ER and bind to Wntless (WLS) for intracellular transport and secretion. Mechanisms governing the transfer of these low-solubility Wnts from the ER to the extracellular space remain unclear. Through structural and functional analyses of Wnt7a, a crucial Wnt involved in central nervous system angiogenesis and blood-brain barrier maintenance, we have elucidated the principles of Wnt biogenesis and Wnt7-specific signaling. The Wnt7a-WLS complex binds to calreticulin (CALR), revealing that CALR functions as a chaperone to facilitate Wnt transfer from PORCN to WLS during Wnt biogenesis. Our structures, functional analyses, and molecular dynamics simulations demonstrate that a phospholipid in the core of Wnt-bound WLS regulates the association and dissociation between Wnt and WLS, suggesting a lipid-mediated Wnt secretion mechanism. Finally, the structure of Wnt7a bound to RECK, a cell-surface Wnt7 co-receptor, reveals how RECK CC4 engages the N-terminal domain of Wnt7a to activate Wnt7-specific signaling.
Organizational Affiliation:
Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA. Electronic address: xiaofeng.qi@utsouthwestern.edu.