Interaction between host G3BP and viral nucleocapsid protein regulates SARS-CoV-2 replication and pathogenicity.
Yang, Z., Johnson, B.A., Meliopoulos, V.A., Ju, X., Zhang, P., Hughes, M.P., Wu, J., Koreski, K.P., Clary, J.E., Chang, T.C., Wu, G., Hixon, J., Duffner, J., Wong, K., Lemieux, R., Lokugamage, K.G., Alvarado, R.E., Crocquet-Valdes, P.A., Walker, D.H., Plante, K.S., Plante, J.A., Weaver, S.C., Kim, H.J., Meyers, R., Schultz-Cherry, S., Ding, Q., Menachery, V.D., Taylor, J.P.(2024) Cell Rep 43: 113965-113965
- PubMed: 38492217
- DOI: https://doi.org/10.1016/j.celrep.2024.113965
- Primary Citation of Related Structures:
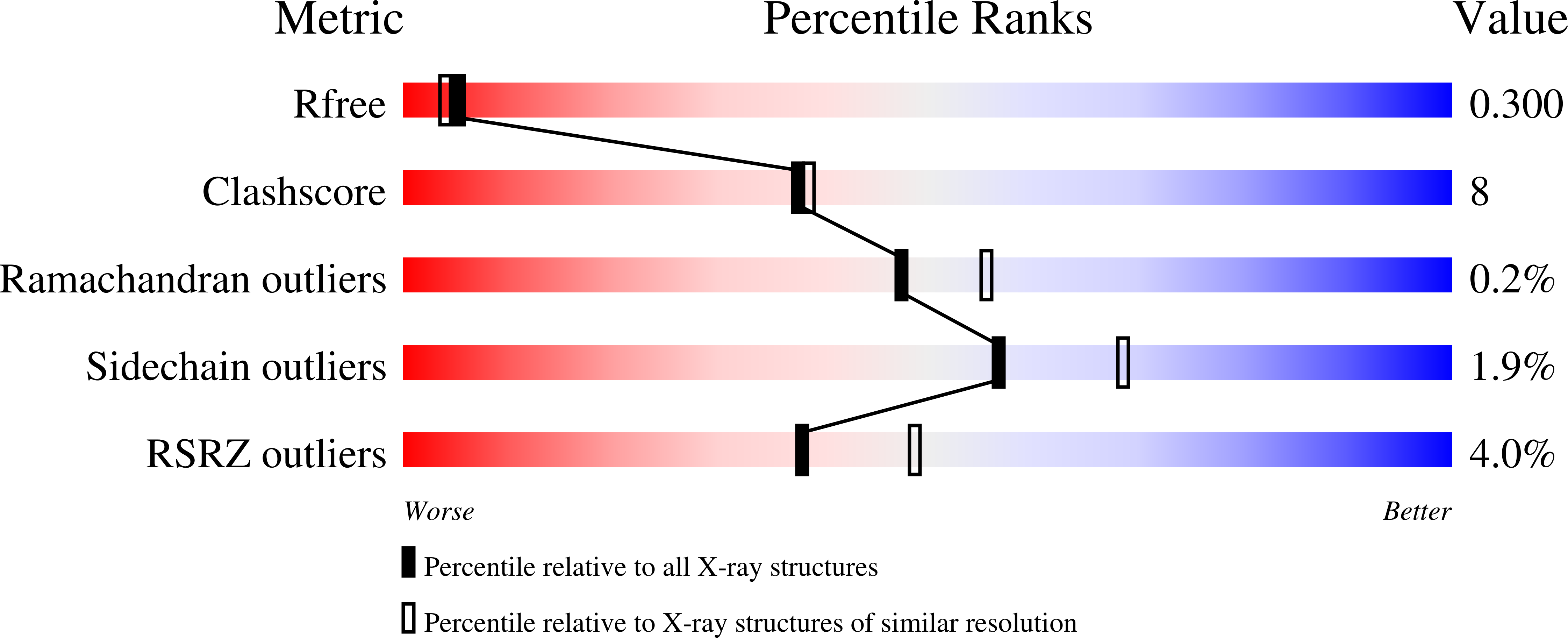
8TH1, 8TH5, 8TH6, 8TH7 - PubMed Abstract:
G3BP1/2 are paralogous proteins that promote stress granule formation in response to cellular stresses, including viral infection. The nucleocapsid (N) protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) inhibits stress granule assembly and interacts with G3BP1/2 via an ITFG motif, including residue F17, in the N protein. Prior studies examining the impact of the G3PB1-N interaction on SARS-CoV-2 replication have produced inconsistent findings, and the role of this interaction in pathogenesis is unknown. Here, we use structural and biochemical analyses to define the residues required for G3BP1-N interaction and structure-guided mutagenesis to selectively disrupt this interaction. We find that N-F17A mutation causes highly specific loss of interaction with G3BP1/2. SARS-CoV-2 N-F17A fails to inhibit stress granule assembly in cells, has decreased viral replication, and causes decreased pathology in vivo. Further mechanistic studies indicate that the N-F17-mediated G3BP1-N interaction promotes infection by limiting sequestration of viral genomic RNA (gRNA) into stress granules.
Organizational Affiliation:
Department of Cell and Molecular Biology, St. Jude Children's Research Hospital, Memphis, TN, USA; Integrated Biomedical Sciences Program, University of Tennessee Health Science Center, Memphis, TN, USA.