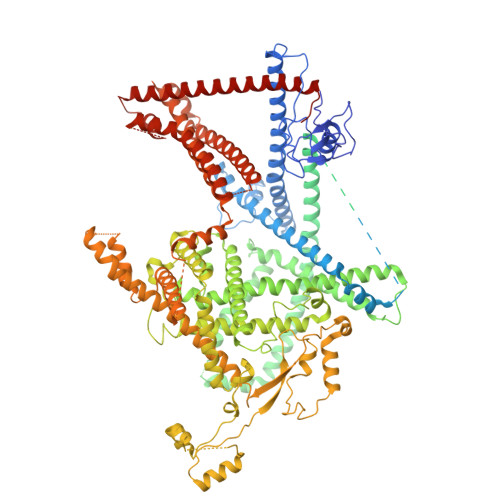
Doa10/MARCH6 architecture interconnects E3 ligase activity with lipid-binding transmembrane channel to regulate SQLE.
Botsch, J.J., Junker, R., Sorgenfrei, M., Ogger, P.P., Stier, L., von Gronau, S., Murray, P.J., Seeger, M.A., Schulman, B.A., Brauning, B.(2024) Nat Commun 15: 410-410
- PubMed: 38195637
- DOI: https://doi.org/10.1038/s41467-023-44670-5
- Primary Citation of Related Structures:
8PD0, 8PDA - PubMed Abstract:
Transmembrane E3 ligases play crucial roles in homeostasis. Much protein and organelle quality control, and metabolic regulation, are determined by ER-resident MARCH6 E3 ligases, including Doa10 in yeast. Here, we present Doa10/MARCH6 structural analysis by cryo-EM and AlphaFold predictions, and a structure-based mutagenesis campaign. The majority of Doa10/MARCH6 adopts a unique circular structure within the membrane. This channel is established by a lipid-binding scaffold, and gated by a flexible helical bundle. The ubiquitylation active site is positioned over the channel by connections between the cytosolic E3 ligase RING domain and the membrane-spanning scaffold and gate. Here, by assaying 95 MARCH6 variants for effects on stability of the well-characterized substrate SQLE, which regulates cholesterol levels, we reveal crucial roles of the gated channel and RING domain consistent with AlphaFold-models of substrate-engaged and ubiquitylation complexes. SQLE degradation further depends on connections between the channel and RING domain, and lipid binding sites, revealing how interconnected Doa10/MARCH6 elements could orchestrate metabolic signals, substrate binding, and E3 ligase activity.
Organizational Affiliation:
Department of Molecular Machines and Signaling, Max Planck Institute of Biochemistry, Am Klopferspitz 18, 82152, Martinsried, Germany.