Structural analyses of the GI.4 norovirus by cryo-electron microscopy and X-ray crystallography revealing binding sites for human monoclonal antibodies.
Kimura-Someya, T., Katsura, K., Kato-Murayama, M., Hosaka, T., Uchikubo-Kamo, T., Ihara, K., Hanada, K., Sato, S., Murayama, K., Kataoka, M., Shirouzu, M., Someya, Y.(2024) J Virol : e0019724-e0019724
- PubMed: 38593321
- DOI: https://doi.org/10.1128/jvi.00197-24
- Primary Citation of Related Structures:
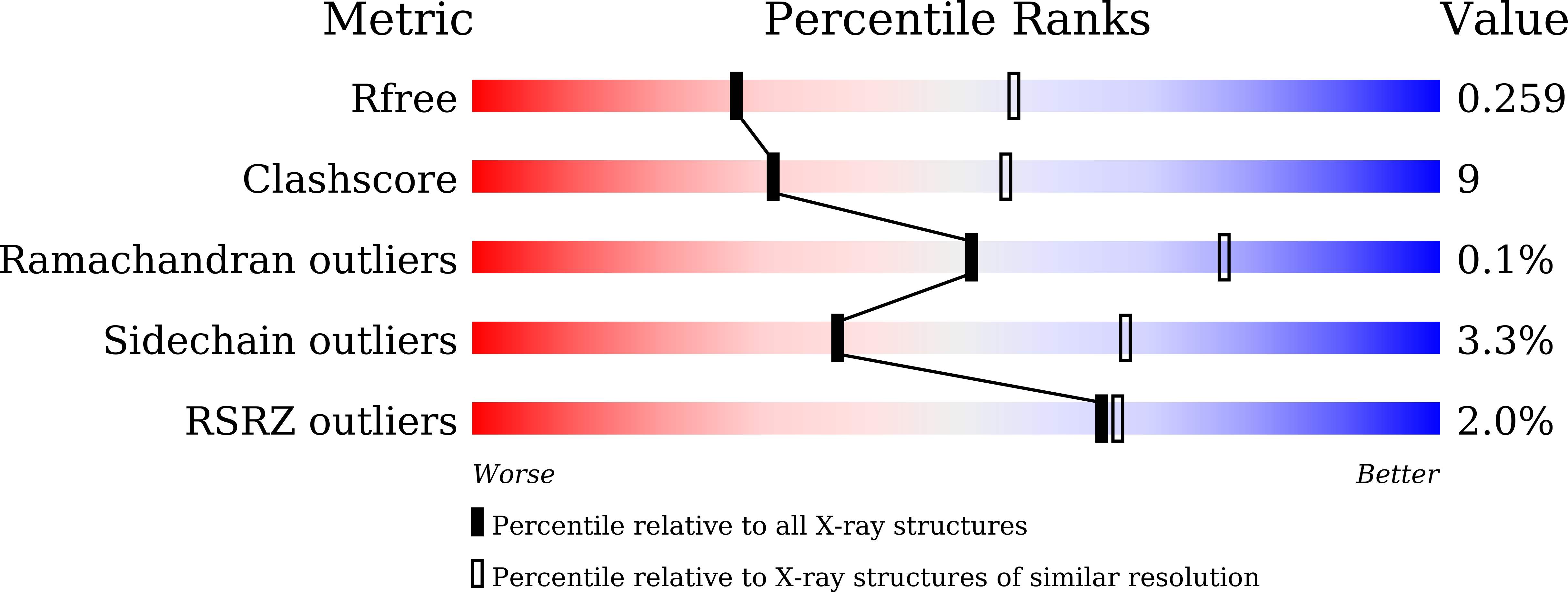
8I5L - PubMed Abstract:
Noroviruses are major causative agents of acute nonbacterial gastroenteritis in humans. There are neither antiviral therapeutic agents nor vaccines for noroviruses at this time. To evaluate the potential usefulness of two previously isolated human monoclonal antibody fragments, CV-1A1 and CV-2F5, we first conducted a single-particle analysis to determine the cryo-electron microscopy structure of virus-like particles (VLPs) from the genogroup I genotype 4 (GI.4) Chiba strain uniformly coated with CV-1A1 fragments. The results revealed that the GI.4-specific CV-1A1 antibody bound to the P2 subdomain, in which amino acids are less conserved and variable. Interestingly, a part of the CV-1A1 intrudes into the histo-blood group antigen-binding site, suggesting that this antibody might exert neutralizing activity. Next, we determined the crystal structure of the protruding (P) domain of the capsid protein in the complex form with the CV-2F5 antibody fragment. Consistent with the cross-reactivity, the CV-2F5 bound to the P1 subdomain, which is rich in amino acids conserved among the GI strains, and moreover induced a disruption of Chiba VLPs. These results suggest that the broadly reactive CV-2F5 antibody can be used as both a universal detection reagent and an antiviral drug for GI noroviruses. We conducted the structural analyses of the VP1 protein from the GI.4 Chiba norovirus to identify the binding sites of the previously isolated human monoclonal antibodies CV-1A1 and CV-2F5. The cryo-electron microscopy of the Chiba virus-like particles (VLPs) complexed with the Fv-clasp forms of GI.4-specific CV-1A1 revealed that this antibody binds to the highly variable P2 subdomain, suggesting that this antibody may have neutralizing ability against the GI.4 strains. X-ray crystallography revealed that the CV-2F5 antibody bound to the P1 subdomain, which is rich in conserved amino acids. This result is consistent with the ability of the CV-2F5 antibody to react with a wide variety of GI norovirus strains. It is also found that the CV-2F5 antibody caused a disruption of VLPs. Our findings, together with previous reports on the structures of VP1 proteins and VLPs, are expected to open a path for the structure-based development of antivirals and vaccines against norovirus disease.
Organizational Affiliation:
RIKEN Center for Biosystems Dynamics Research, Yokohama, Kanagawa, Japan.