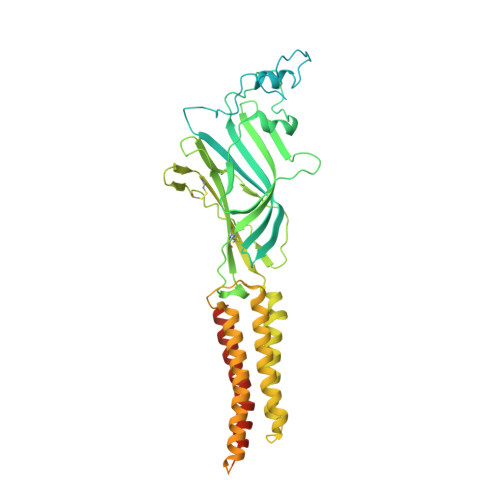
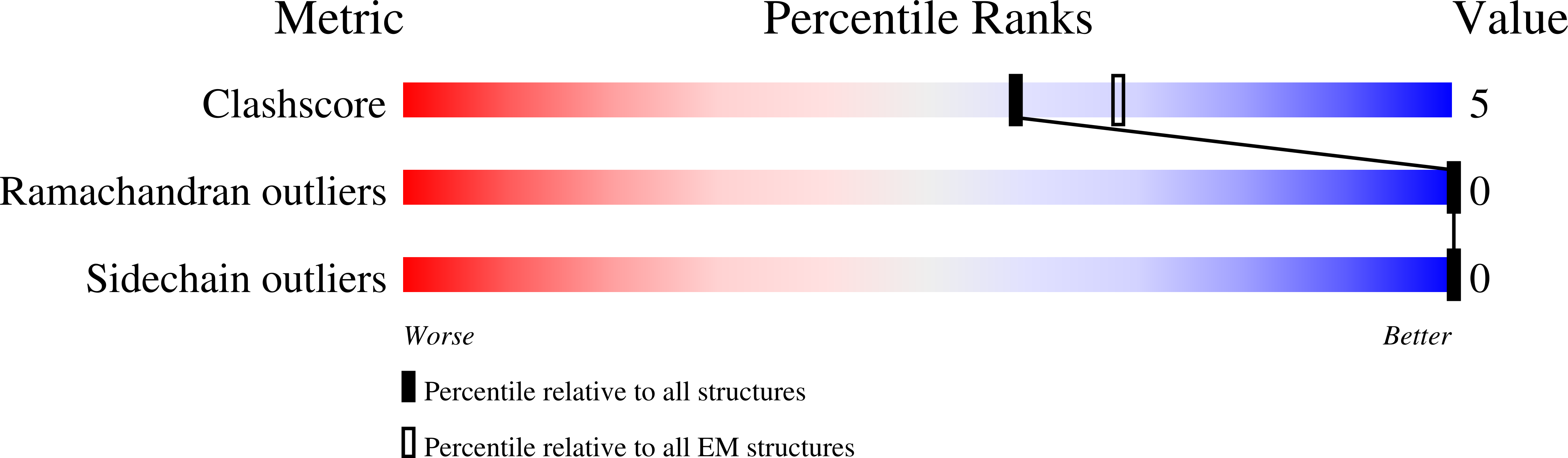Conformational transitions and allosteric modulation in a heteromeric glycine receptor.
Gibbs, E., Klemm, E., Seiferth, D., Kumar, A., Ilca, S.L., Biggin, P.C., Chakrapani, S.(2023) Nat Commun 14: 1363-1363
- PubMed: 36914669
- DOI: https://doi.org/10.1038/s41467-023-37106-7
- Primary Citation of Related Structures:
8FE1 - PubMed Abstract:
Glycine Receptors (GlyRs) provide inhibitory neuronal input in the spinal cord and brainstem, which is critical for muscle coordination and sensory perception. Synaptic GlyRs are a heteromeric assembly of α and β subunits. Here we present cryo-EM structures of full-length zebrafish α1β B GlyR in the presence of an antagonist (strychnine), agonist (glycine), or agonist with a positive allosteric modulator (glycine/ivermectin). Each structure shows a distinct pore conformation with varying degrees of asymmetry. Molecular dynamic simulations found the structures were in a closed (strychnine) and desensitized states (glycine and glycine/ivermectin). Ivermectin binds at all five interfaces, but in a distinct binding pose at the β-α interface. Subunit-specific features were sufficient to solve structures without a fiduciary marker and to confirm the 4α:1β stoichiometry recently observed. We also report features of the extracellular and intracellular domains. Together, our results show distinct compositional and conformational properties of α 1 βGlyR and provide a framework for further study of this physiologically important channel.
Organizational Affiliation:
Department of Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, 44106-4970, USA.