Structural Basis for the Ribonuclease Activity of a Thermostable CRISPR-Cas13a from Thermoclostridium caenicola.
Wang, F., Zhang, C., Xu, H., Zeng, W., Ma, L., Li, Z.(2023) J Mol Biol 435: 168197-168197
- PubMed: 37442412
- DOI: https://doi.org/10.1016/j.jmb.2023.168197
- Primary Citation of Related Structures:
8EWG, 8H4U - PubMed Abstract:
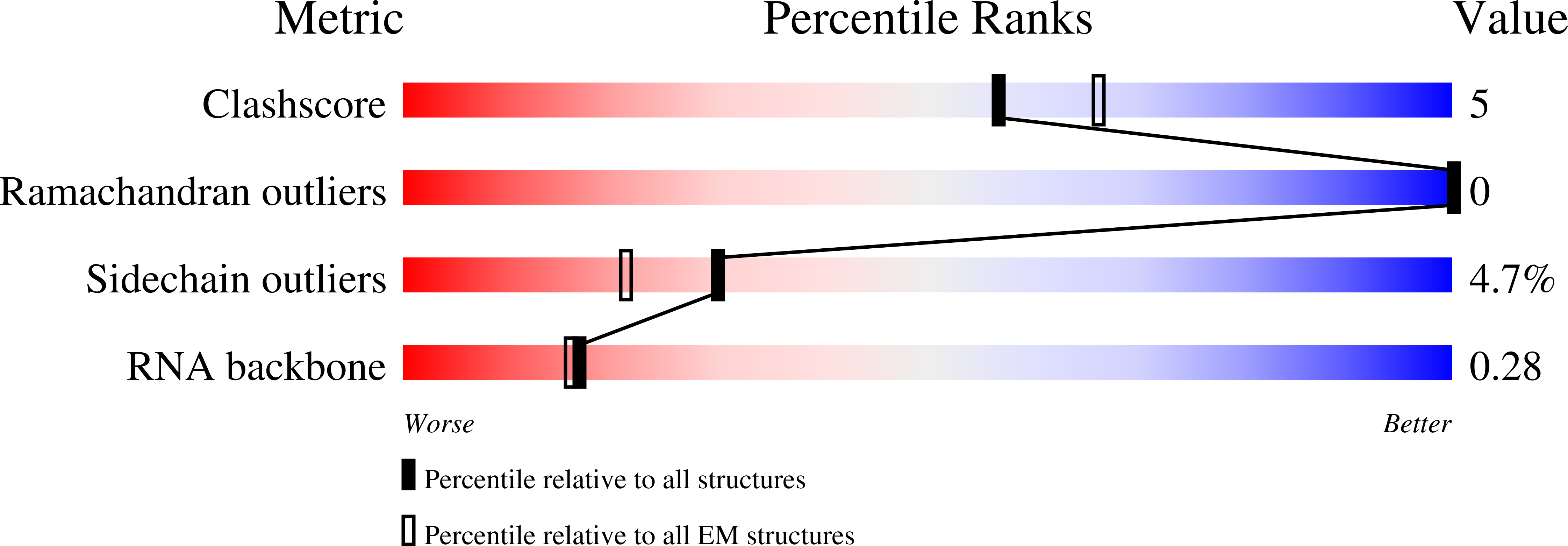
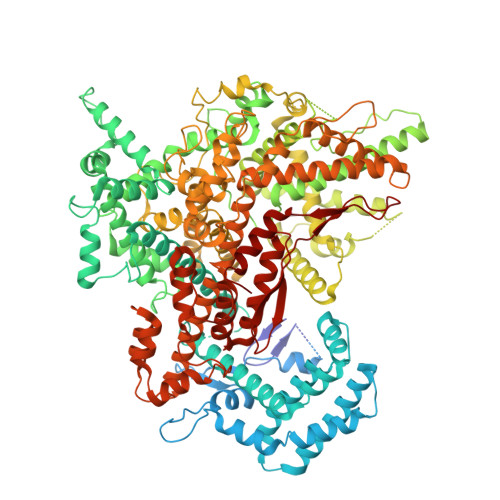
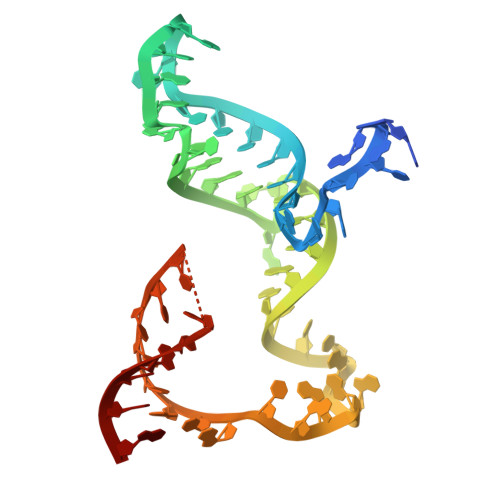
The RNA-targeting type VI CRISPR-Cas effector complexes are widely used in biotechnology applications such as gene knockdown, RNA editing, and molecular diagnostics. Compared with Cas13a from mesophilic organisms, a newly discovered Cas13a from thermophilic bacteria Thermoclostridium caenicola (TccCas13a) shows low sequence similarity, high thermostability, and lacks pre-crRNA processing activity. The thermostability of TccCas13a has been harnessed to make a sensitive and robust tool for nucleic acid detection. Here we present the structures of TccCas13a-crRNA binary complex at 2.8 Å, and TccCas13a at 3.5 Å. Although TccCas13a shares a similarly bilobed architecture with other mesophilic organism-derived Cas13a proteins, TccCas13a displayed distinct structure features. Specifically, it holds a long crRNA 5'-flank, forming extensive polar contacts with Helical-1 and HEPN2 domains. The detailed analysis of the interaction between crRNA 5'-flank and TccCas13a suggested lack of suitable nucleophile to attack the 2'-OH of crRNA 5'-flank may explain why TccCas13a fails to cleave pre-crRNA. The stem-loop segment of crRNA spacer toggles between double-stranded and single-stranded conformational states, suggesting a potential safeguard mechanism for target recognition. Superimposition of the structures of TccCas13a and TccCas13a-crRNA revealed several conformational changes required for crRNA loading, including dramatic movement of Helical-2 domain. Collectively, these structural insights expand our understanding into type VI CRISPR-Cas effectors, and would facilitate the development of TccCas13a-based applications.
Organizational Affiliation:
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei 430062, China.