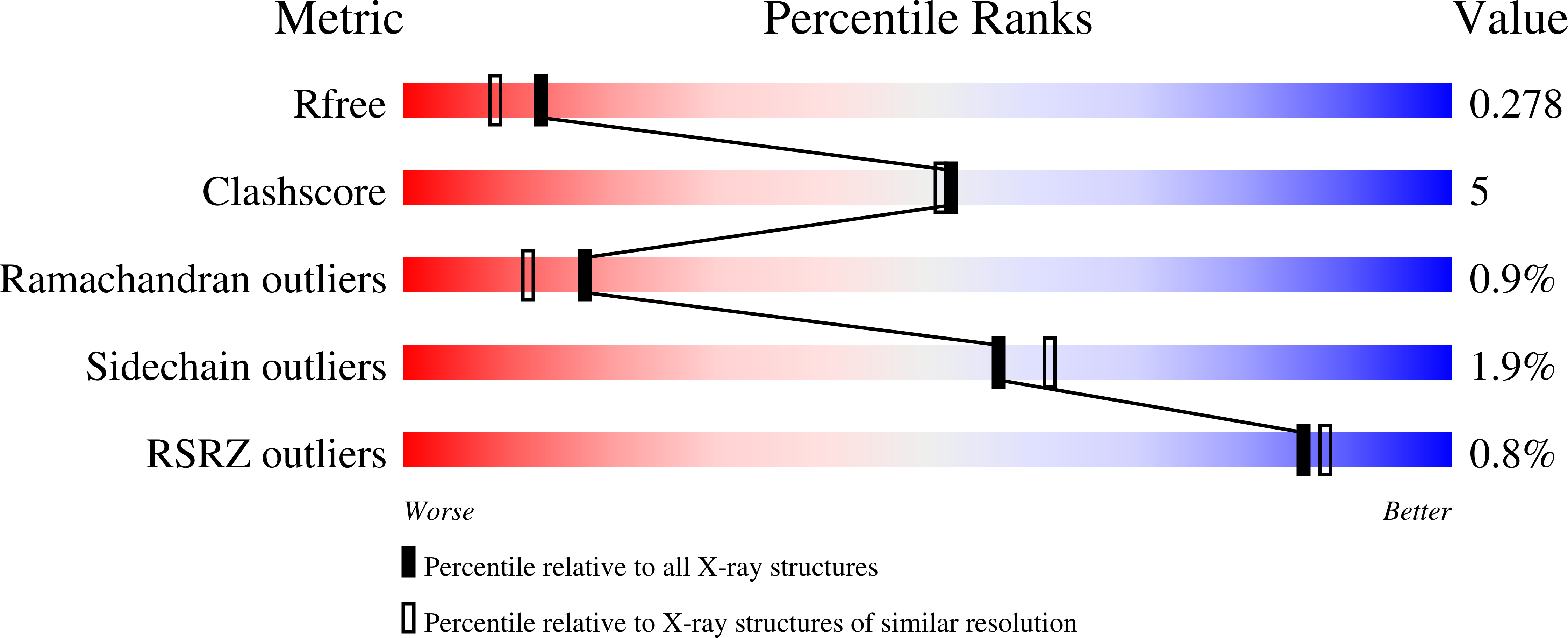
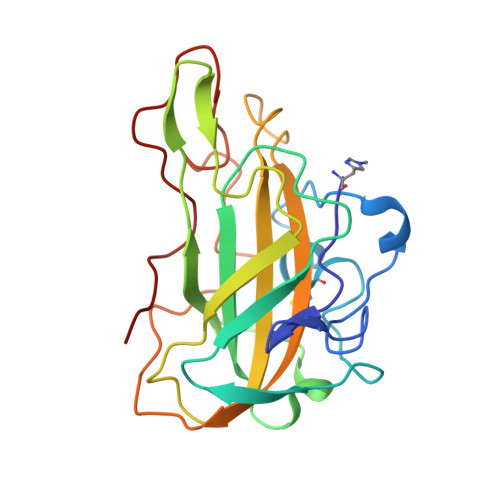
Characterisation of lytic polysaccharide monooxygenases from Aspergillus nidulans
Rafael Fanchini Terrasan, C., Males, A., Figueiredo, F.L., Gerhardt, J.A., Rubio, M.V., Franco Cairo, J.P., Lindley, P., Steward, M., Lima Valadares, F., Correa, T.L.R., Davies, G.J., Damasio, A., Walton, P.H.To be published.