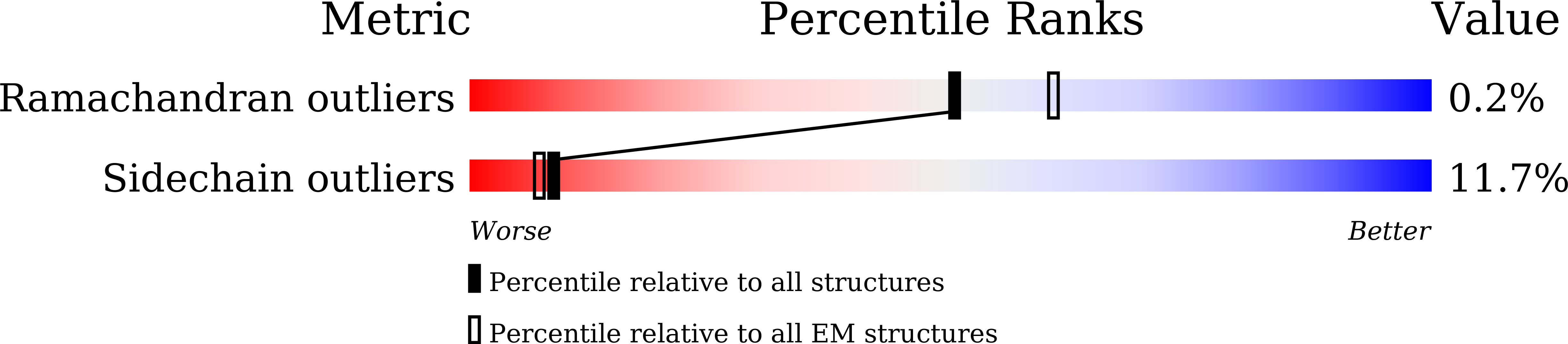Architecture of the bacteriophage lambda tail.
Wang, C., Duan, J., Gu, Z., Ge, X., Zeng, J., Wang, J.(2024) Structure 32: 35
- PubMed: 37918400
- DOI: https://doi.org/10.1016/j.str.2023.10.006
- Primary Citation of Related Structures:
8IYD, 8IYK, 8IYL, 8JVM, 8KGE - PubMed Abstract:
Bacteriophage lambda has a double-stranded DNA genome and a long, flexible, non-contractile tail encoded by a contiguous block of 11 genes downstream of the head genes. The tail allows host recognition and delivery of viral DNA from the head shell to the cytoplasm of the infected cell. Here, we present a high-resolution structure of the tail complex of bacteriophage lambda determined by cryoelectron microscopy. Most component proteins of the lambda tail were determined at the atomic scale. The structure sheds light on the molecular organization of the extensively studied tail of bacteriophage lambda.
Organizational Affiliation:
State Key Laboratory of Membrane Biology, Beijing Frontier Research Center for Biological Structure, School of Life Sciences, Tsinghua University, Beijing 100084, P.R. China.