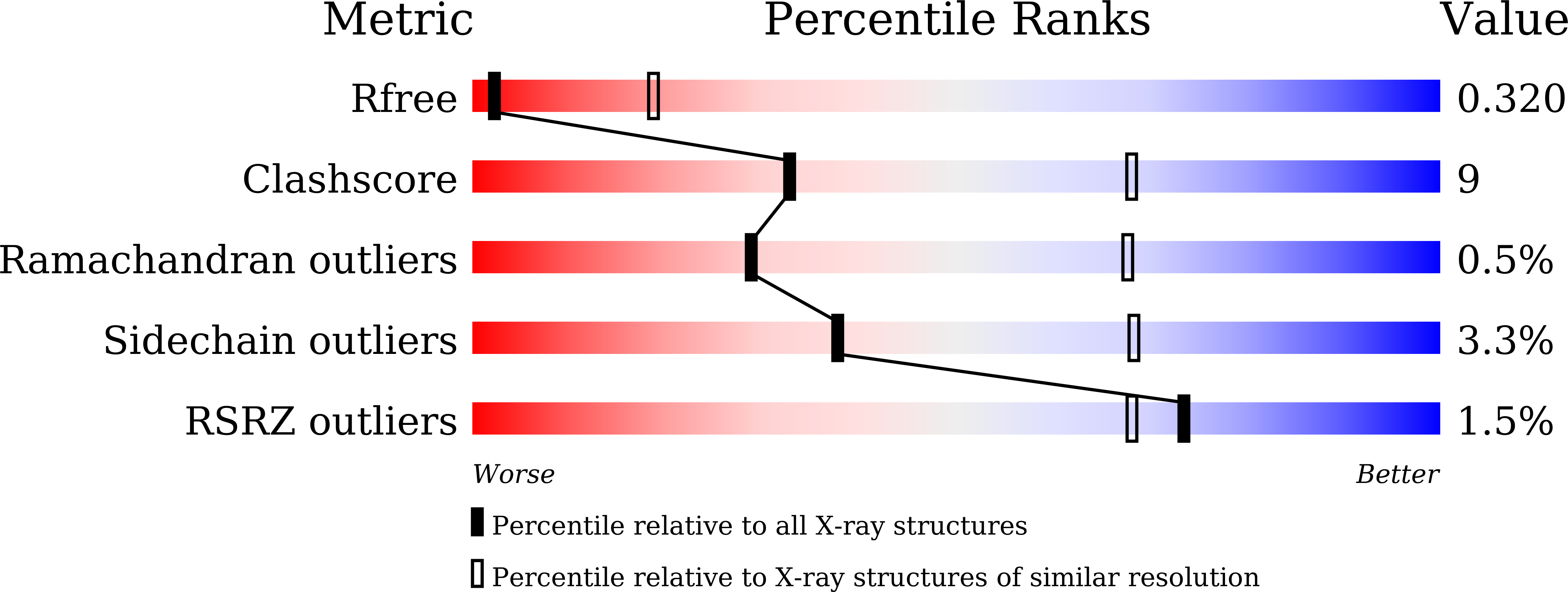
Structure-function analysis of bacterial GH31 alpha-galactosidases specific for alpha-(1→4)-galactobiose.
Ikegaya, M., Park, E.Y., Miyazaki, T.(2023) FEBS J 290: 4984-4998
- PubMed: 37438884
- DOI: https://doi.org/10.1111/febs.16904
- Primary Citation of Related Structures:
8J50, 8J51, 8J52, 8J53 - PubMed Abstract:
Glycoside hydrolase family 31 (GH31) contains α-glycoside hydrolases with different substrate specificities involved in various physiological functions. This family has recently been classified into 20 subfamilies using sequence similarity networks. An α-galactosidase from the gut bacterium Bacteroides salyersiae (BsGH31_19, which belongs to GH31 subfamily 19) was reported to have hydrolytic activity against the synthetic substrate p- nitrophenyl α-galactopyranoside, but its natural substrate remained unknown. BsGH31_19 shares low sequence identity (around 20%) with other reported GH31 α-galactosidases, PsGal31A from Pseudopedobacter saltans and human myogenesis-regulating glycosidase (MYORG), and was expected to have distinct specificity. Here, we characterized BsGH31_19 and its ortholog from a soil Bacteroidota bacterium, Flavihumibacter petaseus (FpGH31_19), and demonstrated that they showed high substrate specificity against α-(1→4)-linkages in α-(1→4)-galactobiose and globotriose [α-Gal-(1→4)-β-Gal-(1→4)-Glc], unlike PsGal31A and MYORG. The crystallographic analyses of BsGH31_19 and FpGH31_19 showed that their overall structures resemble those of MYORG and form a dimer with an interface different from that of PsGal31A and MYORG dimers. The structures of FpGH31_19 complexed with d-galactose and α-(1→4)-galactobiose revealed that amino acid residues that recognize a galactose residue at subsite +1 are not conserved between FpGH31_19 and BsGH31_19. The tryptophan (Trp153) that recognizes galactose at subsite -1 is homologous to the tryptophan residues in MYORG and α-galactosidases belonging to GH27, GH36, and GH97, but not in the bacterial GH31 member PsGal31A. Our results provide structural insights into molecular diversity and evolutionary relationships in the GH31 α-galactosidase subfamilies and the other α-galactosidase families.
Organizational Affiliation:
Department of Bioscience, Graduate School of Science and Technology, Shizuoka University, Japan.