Crystal Structure of 4-hydroxybutyryl CoA synthetase (ADP-forming): A Key Enzyme in the Thaumarchaeal Hydroxypropionate/Hydroxybutyrate cycle.
Johnson, J., Demirci, H.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 4-hydroxybutyrate--CoA ligase [ADP-forming] | 698 | Nitrosopumilus maritimus SCM1 | Mutation(s): 0 Gene Names: Nmar_0206 EC: 6.2.1.56 | 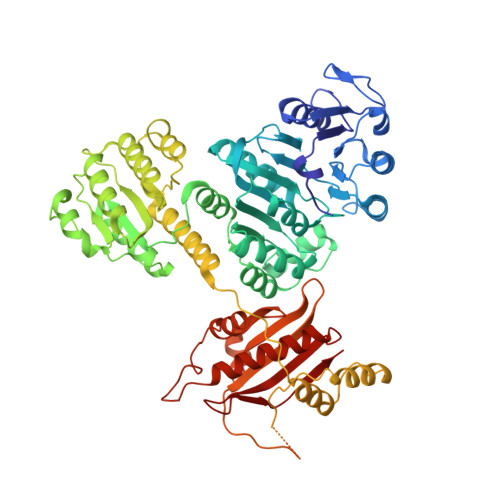 | |
UniProt | |||||
Find proteins for A9A1Y1 (Nitrosopumilus maritimus (strain SCM1)) Explore A9A1Y1 Go to UniProtKB: A9A1Y1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A9A1Y1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 (Subject of Investigation/LOI) Query on SO4 | C [auth A], D [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 356.98 | α = 90 |
| b = 70.4 | β = 90 |
| c = 75.81 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Foundation (NSF, United States) | United States | 1231306 |