The Effect of Deuteration and Homologation of the Lactam Ring of Nirmatrelvir on Its Biochemical Properties and Oxidative Metabolism.
Arutyunova, E., Belovodskiy, A., Chen, P., Khan, M.B., Joyce, M., Saffran, H., Lu, J., Turner, Z., Bai, B., Lamer, T., Young, H.S., Vederas, J.C., Tyrrell, D.L., Lemieux, M.J., Nieman, J.A.(2023) ACS Bio Med Chem Au 3: 528-541
- PubMed: 38144257
- DOI: https://doi.org/10.1021/acsbiomedchemau.3c00039
- Primary Citation of Related Structures:
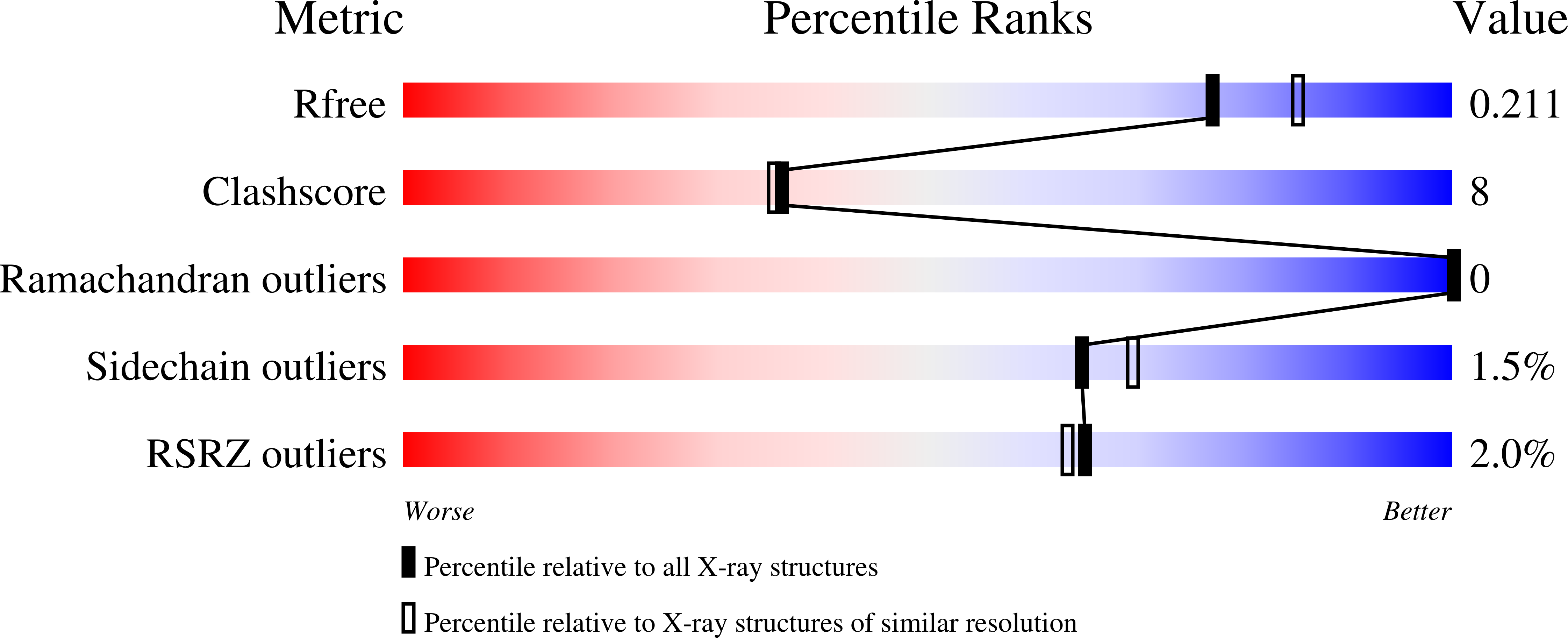
8FTC - PubMed Abstract:
This study explores the relationship between structural alterations of nirmatrelvir, such as homologation and deuteration, and metabolic stability of newly synthesized derivatives. We developed a reliable synthetic protocol toward dideutero-nirmatrelvir and its homologated analogues with high isotopic incorporation. Deuteration of the primary metabolic site of nirmatrelvir provides a 3-fold improvement of its human microsomal stability but is accompanied by an increased metabolism rate at secondary sites. Homologation of the lactam ring allows the capping group modification to decrease and delocalize the molecule's lipophilicity, reducing the metabolic rate at secondary sites. The effect of deuteration was less pronounced for the 6-membered lactam than for its 5-membered analogue in human microsomes, but the trend is reversed in the case of mouse microsomes. X-ray data revealed that the homologation of the lactam ring favors the orientation of the drug's nitrile warhead for interaction with the catalytic sulfur of the SARS-CoV-2 M pro , improving its binding. Comparable potency against SARS-CoV-2 M pro from several variants of concern and selectivity over human cysteine proteases cathepsin B, L, and S was observed for the novel deuterated/homologated derivative and nirmatrelvir. Synthesized compounds displayed a large interspecies variability in hamster, rat, and human hepatocyte stability assays. Overall, we aimed to apply a rational approach in changing the physicochemical properties of the drug to refine its biochemical and biological parameters.
Organizational Affiliation:
Department of Biochemistry, University of Alberta, Edmonton, AB T6G 2H7, Canada.