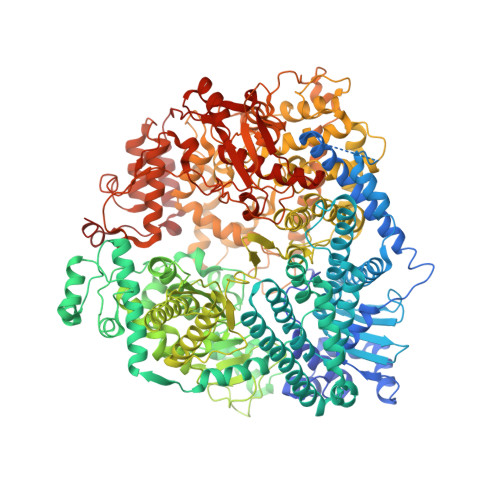
Conserved allosteric inhibitory site on the respiratory syncytial virus and human metapneumovirus RNA-dependent RNA polymerases.
Kleiner, V.A., Fischmann, T.O., Howe, J.A., Beshore, D.C., Eddins, M.J., Hou, Y., Mayhood, T., Klein, D., Nahas, D.D., Lucas, B.J., Xi, H., Murray, E., Ma, D.Y., Getty, K., Fearns, R.(2023) Commun Biol 6: 649-649
- PubMed: 37337079
- DOI: https://doi.org/10.1038/s42003-023-04990-0
- Primary Citation of Related Structures:
8FPI, 8FPJ - PubMed Abstract:
Respiratory syncytial virus (RSV) and human metapneumovirus (HMPV) are related RNA viruses responsible for severe respiratory infections and resulting disease in infants, elderly, and immunocompromised adults 1-3 . Therapeutic small molecule inhibitors that bind to the RSV polymerase and inhibit viral replication are being developed, but their binding sites and molecular mechanisms of action remain largely unknown 4 . Here we report a conserved allosteric inhibitory site identified on the L polymerase proteins of RSV and HMPV that can be targeted by a dual-specificity, non-nucleoside inhibitor, termed MRK-1. Cryo-EM structures of the inhibitor in complexes with truncated RSV and full-length HMPV polymerase proteins provide a structural understanding of how MRK-1 is active against both viruses. Functional analyses indicate that MRK-1 inhibits conformational changes necessary for the polymerase to engage in RNA synthesis initiation and to transition into an elongation mode. Competition studies reveal that the MRK-1 binding pocket is distinct from that of a capping inhibitor with an overlapping resistance profile, suggesting that the polymerase conformation bound by MRK-1 may be distinct from that involved in mRNA capping. These findings should facilitate optimization of dual RSV and HMPV replication inhibitors and provide insights into the molecular mechanisms underlying their polymerase activities.
Organizational Affiliation:
Department of Virology, Immunology & Microbiology, National Emerging Infectious Diseases Laboratories, Boston University Chobanian and Avedisian School of Medicine, Boston, MA, USA.