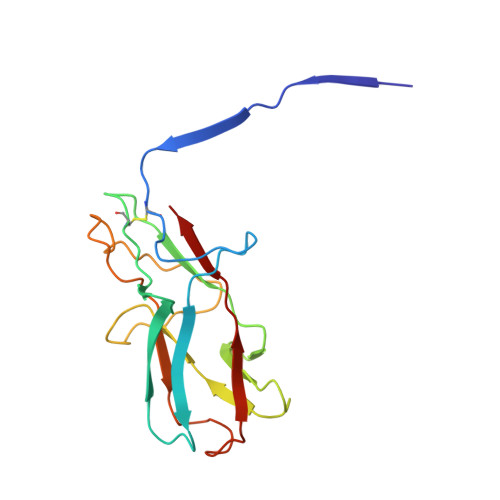
Architecture of the biofilm-associated archaic Chaperone-Usher pilus CupE from Pseudomonas aeruginosa.
Bohning, J., Dobbelstein, A.W., Sulkowski, N., Eilers, K., von Kugelgen, A., Tarafder, A.K., Peak-Chew, S.Y., Skehel, M., Alva, V., Filloux, A., Bharat, T.A.M.(2023) PLoS Pathog 19: e1011177-e1011177
- PubMed: 37058467
- DOI: https://doi.org/10.1371/journal.ppat.1011177
- Primary Citation of Related Structures:
8CIO - PubMed Abstract:
Chaperone-Usher Pathway (CUP) pili are major adhesins in Gram-negative bacteria, mediating bacterial adherence to biotic and abiotic surfaces. While classical CUP pili have been extensively characterized, little is known about so-called archaic CUP pili, which are phylogenetically widespread and promote biofilm formation by several human pathogens. In this study, we present the electron cryomicroscopy structure of the archaic CupE pilus from the opportunistic human pathogen Pseudomonas aeruginosa. We show that CupE1 subunits within the pilus are arranged in a zigzag architecture, containing an N-terminal donor β-strand extending from each subunit into the next, where it is anchored by hydrophobic interactions, with comparatively weaker interactions at the rest of the inter-subunit interface. Imaging CupE pili on the surface of P. aeruginosa cells using electron cryotomography shows that CupE pili adopt variable curvatures in response to their environment, which might facilitate their role in promoting cellular attachment. Finally, bioinformatic analysis shows the widespread abundance of cupE genes in isolates of P. aeruginosa and the co-occurrence of cupE with other cup clusters, suggesting interdependence of cup pili in regulating bacterial adherence within biofilms. Taken together, our study provides insights into the architecture of archaic CUP pili, providing a structural basis for understanding their role in promoting cellular adhesion and biofilm formation in P. aeruginosa.
Organizational Affiliation:
Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom.