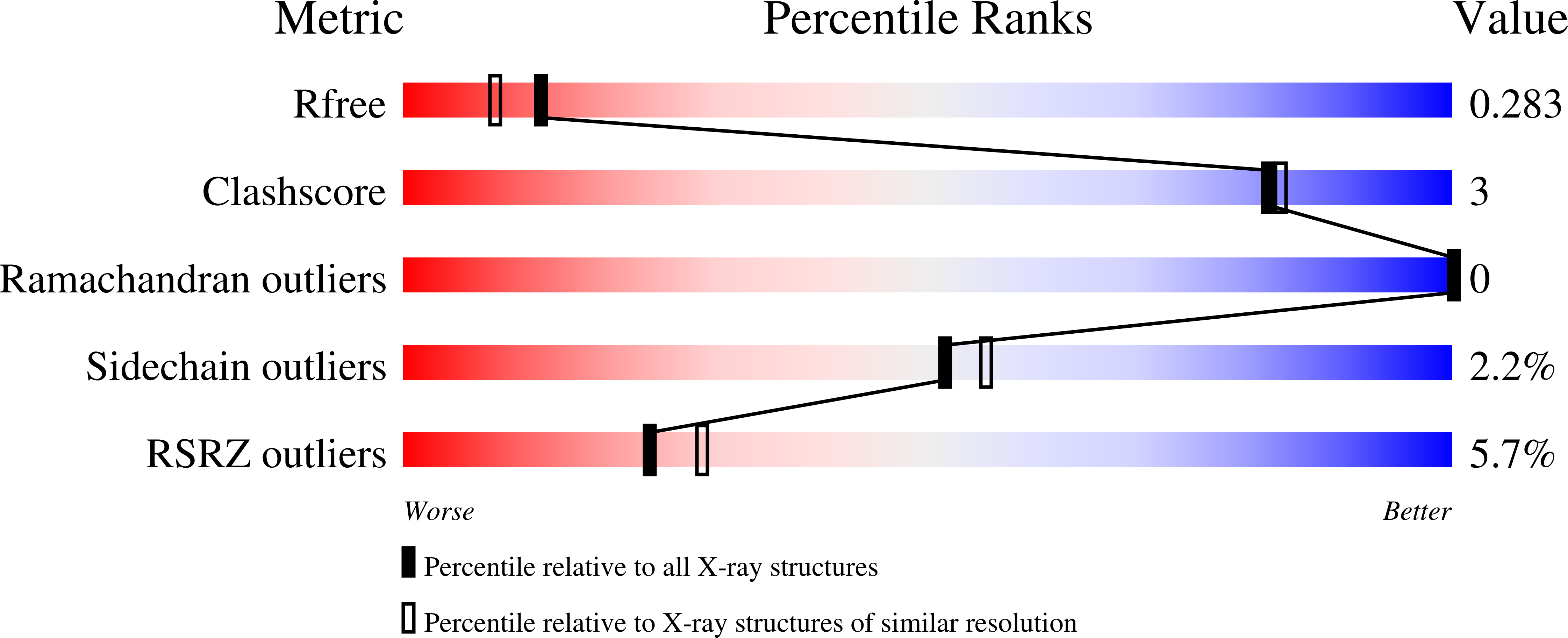
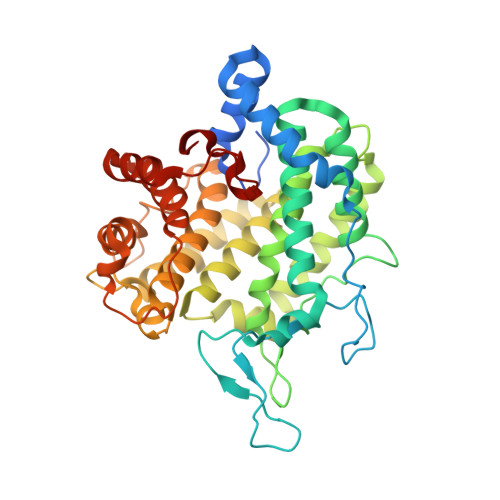
Three alginate lyases provide a new gut Bacteroides ovatus isolate with the ability to grow on alginate.
Ronne, M.E., Tandrup, T., Madsen, M., Hunt, C.J., Myers, P.N., Moll, J.M., Holck, J., Brix, S., Strube, M.L., Aachmann, F.L., Wilkens, C., Svensson, B.(2023) Appl Environ Microbiol 89: e0118523-e0118523
- PubMed: 37791757
- DOI: https://doi.org/10.1128/aem.01185-23
- Primary Citation of Related Structures:
8BDD, 8BDQ - PubMed Abstract:
Humans consume alginate in the form of seaweed, food hydrocolloids, and encapsulations, making the digestion of this mannuronic acid (M) and guluronic acid (G) polymer of key interest for human health. To increase knowledge on alginate degradation in the gut, a gene catalog from human feces was mined for potential alginate lyases (ALs). The predicted ALs were present in nine species of the Bacteroidetes phylum, of which two required supplementation of an endo -acting AL, expected to mimic cross-feeding in the gut. However, only a new isolate grew on alginate. Whole-genome sequencing of this alginate-utilizing isolate suggested that it is a new Bacteroides ovatus strain harboring a polysaccharide utilization locus (PUL) containing three ALs of families: PL6, PL17, and PL38. The Bo PL6 degraded polyG to oligosaccharides of DP 1-3, and Bo PL17 released 4,5-unsaturated monouronate from polyM. Bo PL38 degraded both alginates, polyM, polyG, and polyMG, in endo -mode; hence, it was assumed to deliver oligosaccharide substrates for Bo PL6 and Bo PL17, corresponding well with synergistic action on alginate. Bo PL17 and Bo PL38 crystal structures, determined at 1.61 and 2.11 Å, respectively, showed (α/α) 6 -barrel + anti-parallel β-sheet and (α/α) 7 -barrel folds, distinctive for these PL families. Bo PL17 had a more open active site than the two homologous structures. Bo PL38 was very similar to the structure of an uncharacterized PL38, albeit with a different triad of residues possibly interacting with substrate in the presumed active site tunnel. Altogether, the study provides unique functional and structural insights into alginate-degrading lyases of a PUL in a human gut bacterium.IMPORTANCEHuman ingestion of sustainable biopolymers calls for insight into their utilization in our gut. Seaweed is one such resource with alginate, a major cell wall component, used as a food hydrocolloid and for encapsulation of pharmaceuticals and probiotics. Knowledge is sparse on the molecular basis for alginate utilization in the gut. We identified a new Bacteroides ovatus strain from human feces that grew on alginate and encoded three alginate lyases in a gene cluster. Bo PL6 and Bo PL17 show complementary specificity toward guluronate (G) and mannuronate (M) residues, releasing unsaturated oligosaccharides and monouronic acids. Bo PL38 produces oligosaccharides degraded by Bo PL6 and Bo PL17 from both alginates, G-, M-, and MG-substrates. Enzymatic and structural characterization discloses the mode of action and synergistic degradation of alginate by these alginate lyases. Other bacteria were cross-feeding on alginate oligosaccharides produced by an endo-acting alginate lyase. Hence, there is an interdependent community in our guts that can utilize alginate.
Organizational Affiliation:
Department of Biotechnology and Biomedicine, Enzyme and Protein Chemistry, Technical University of Denmark, Kgs. Lyngby, Denmark.