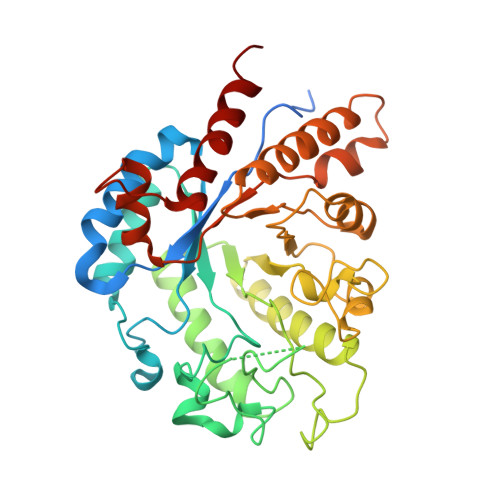
Mechanistic and Structural Insights into the Specificity and Biological Functions of Bacterial Sulfoglycosidases
Zhang, Z., Dong, M., Zallot, R., Blackburn, G.M., Wang, N., Wang, C., Chen, L., Baumann, P., Wu, Z., Wang, Z., Fan, H., Roth, C., Jin, Y., He, Y.(2022) ACS Catal