The Crystal Structure of Bacillus thuringiensis Tpp80Aa1 and Its Interaction with Galactose-Containing Glycolipids.
Best, H.L., Williamson, L.J., Lipka-Lloyd, M., Waller-Evans, H., Lloyd-Evans, E., Rizkallah, P.J., Berry, C.(2022) Toxins (Basel) 14
- PubMed: 36548760
- DOI: https://doi.org/10.3390/toxins14120863
- Primary Citation of Related Structures:
8BAD - PubMed Abstract:
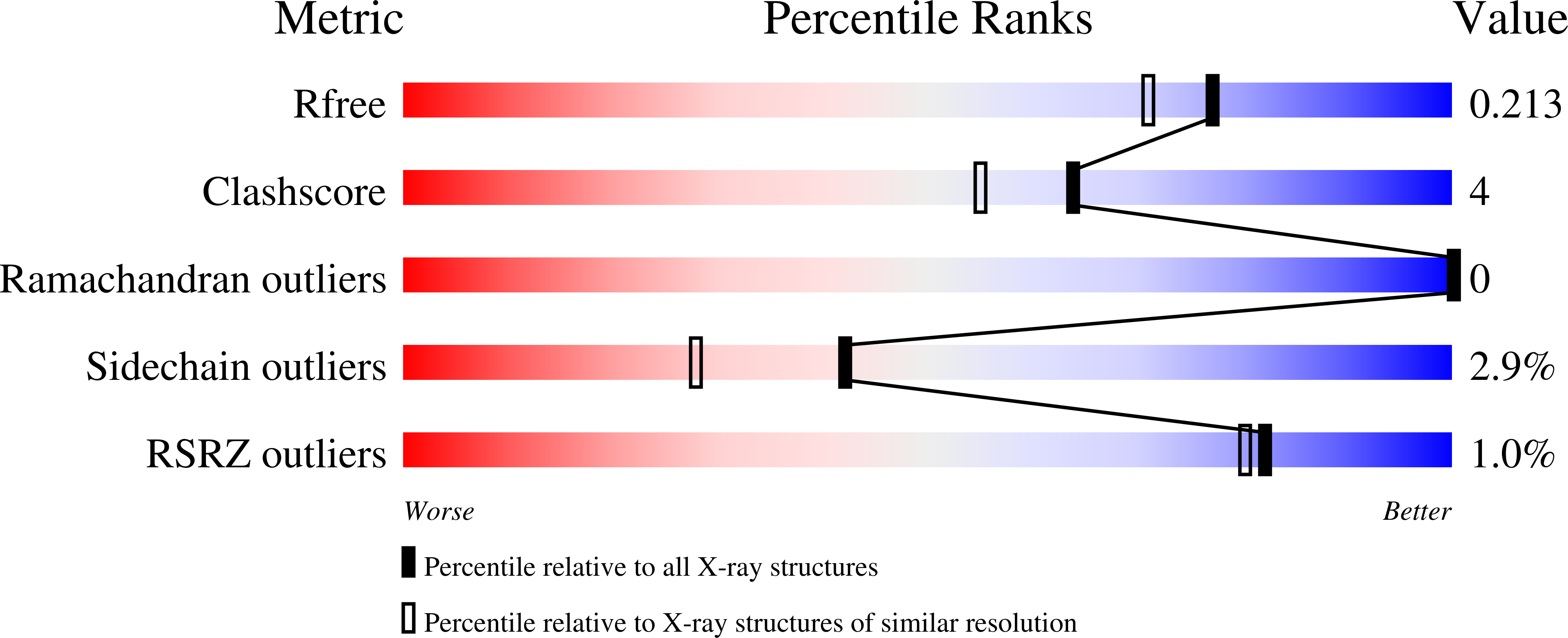
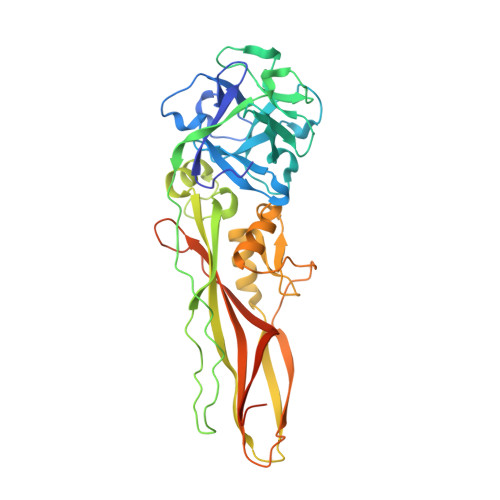
Tpp80Aa1 from Bacillus thuringiensis is a Toxin_10 family protein (Tpp) with reported action against Culex mosquitoes. Here, we demonstrate an expanded target range, showing Tpp80Aa1 is also active against the larvae of Anopheles gambiae and Aedes aegypti mosquitoes. We report the first crystal structure of Tpp80Aa1 at a resolution of 1.8 Å, which shows Tpp80Aa1 consists of two domains: an N-terminal β-trefoil domain resembling a ricin B lectin and a C-terminal putative pore-forming domain sharing structural similarity with the aerolysin family. Similar to other Tpp family members, we observe Tpp80Aa1 binds to the mosquito midgut, specifically the posterior midgut and the gastric caecum. We also identify that Tpp80Aa1 can interact with galactose-containing glycolipids and galactose, and this interaction is critical for exerting full insecticidal action against mosquito target cell lines.
Organizational Affiliation:
School of Biosciences, Cardiff University, Park Place, Cardiff CF10 3AX, UK.