Discovery of N -beta-l-Fucosyl Amides as High-Affinity Ligands for the Pseudomonas aeruginosa Lectin LecB.
Mala, P., Siebs, E., Meiers, J., Rox, K., Varrot, A., Imberty, A., Titz, A.(2022) J Med Chem 65: 14180-14200
- PubMed: 36256875
- DOI: https://doi.org/10.1021/acs.jmedchem.2c01373
- Primary Citation of Related Structures:
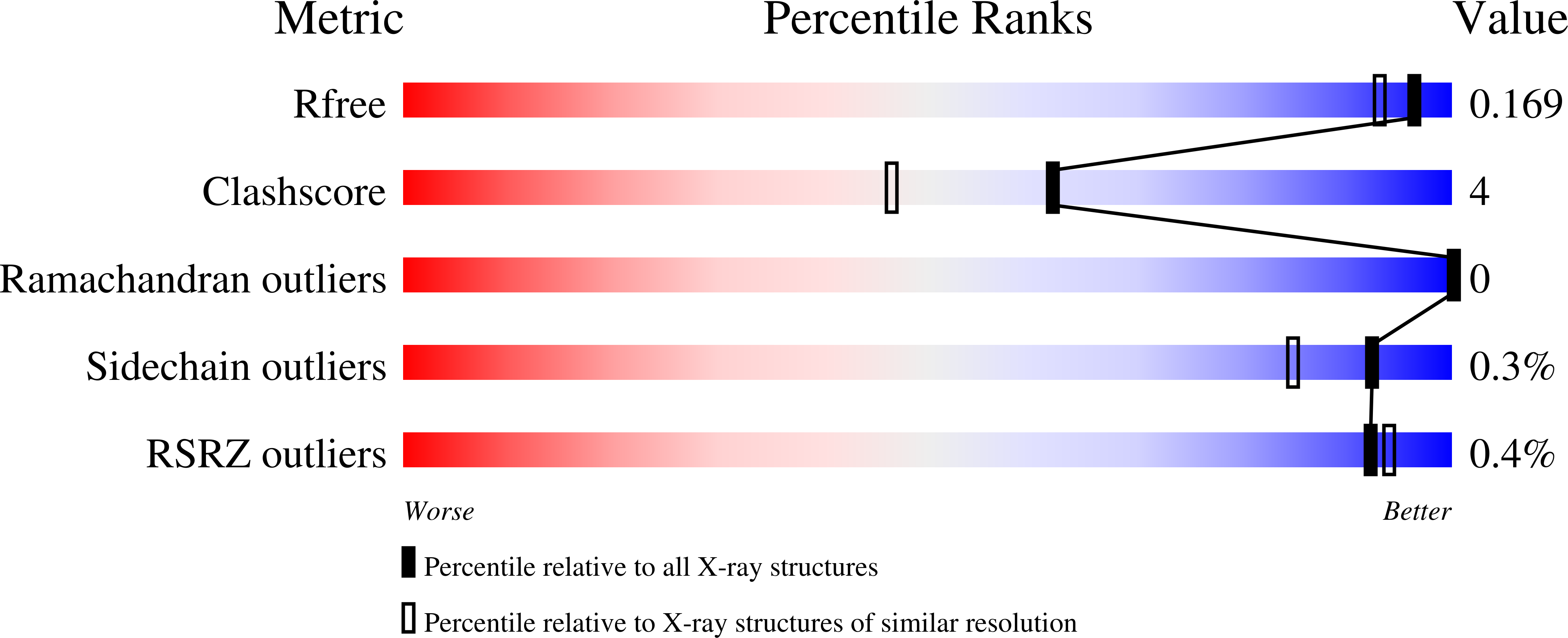
8AIJ, 8AIY - PubMed Abstract:
The Gram-negative pathogen Pseudomonas aeruginosa causes severe infections mainly in immunocompromised or cystic fibrosis patients and is able to resist antimicrobial treatments. The extracellular lectin LecB plays a key role in bacterial adhesion to the host and biofilm formation. For the inhibition of LecB, we designed and synthesized a set of fucosyl amides, sulfonamides, and thiourea derivatives. Then, we analyzed their binding to LecB in competitive and direct binding assays. We identified β-fucosyl amides as unprecedented high-affinity ligands in the two-digit nanomolar range. X-ray crystallography of one α- and one β-anomer of N -fucosyl amides in complex with LecB revealed the interactions responsible for the high affinity of the β-anomer at atomic level. Further, the molecules showed good stability in murine and human blood plasma and hepatic metabolism, providing a basis for future development into antibacterial drugs.
Organizational Affiliation:
Chemical Biology of Carbohydrates (CBCH), Helmholtz-Institute for Pharmaceutical Research Saarland (HIPS), Helmholtz Centre for Infection Research, 66123Saarbrücken, Germany.