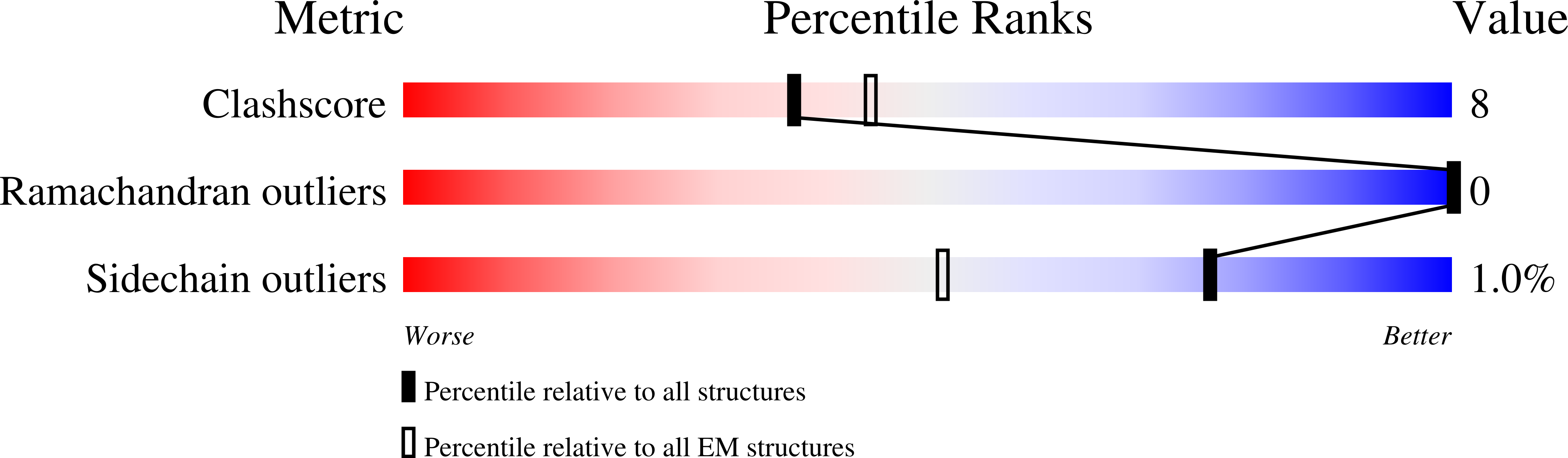Structure and supramolecular organization of the canine distemper virus attachment glycoprotein.
Kalbermatter, D., Jeckelmann, J.M., Wyss, M., Shrestha, N., Pliatsika, D., Riedl, R., Lemmin, T., Plattet, P., Fotiadis, D.(2023) Proc Natl Acad Sci U S A 120: e2208866120-e2208866120
- PubMed: 36716368
- DOI: https://doi.org/10.1073/pnas.2208866120
- Primary Citation of Related Structures:
7ZNY - PubMed Abstract:
Canine distemper virus (CDV) is an enveloped RNA morbillivirus that triggers respiratory, enteric, and high incidence of severe neurological disorders. CDV induces devastating outbreaks in wild and endangered animals as well as in domestic dogs in countries associated with suboptimal vaccination programs. The receptor-binding tetrameric attachment (H)-protein is part of the morbilliviral cell entry machinery. Here, we present the cryo-electron microscopy (cryo-EM) structure and supramolecular organization of the tetrameric CDV H-protein ectodomain. The structure reveals that the morbilliviral H-protein is composed of three main domains: stalk, neck, and heads. The most unexpected feature was the inherent asymmetric architecture of the CDV H-tetramer being shaped by the neck, which folds into an almost 90° bent conformation with respect to the stalk. Consequently, two non-contacting receptor-binding H-head dimers, which are also tilted toward each other, are located on one side of an intertwined four helical bundle stalk domain. Positioning of the four protomer polypeptide chains within the neck domain is guided by a glycine residue (G158), which forms a hinge point exclusively in two protomer polypeptide chains. Molecular dynamics simulations validated the stability of the asymmetric structure under near physiological conditions and molecular docking showed that two receptor-binding sites are fully accessible. Thus, this spatial organization of the CDV H-tetramer would allow for concomitant protein interactions with the stalk and head domains without steric clashes. In summary, the structure of the CDV H-protein ectodomain provides new insights into the morbilliviral cell entry system and offers a blueprint for next-generation structure-based antiviral drug discovery.
Organizational Affiliation:
Institute of Biochemistry and Molecular Medicine, Medical Faculty, University of Bern, CH-3012 Bern, Switzerland.