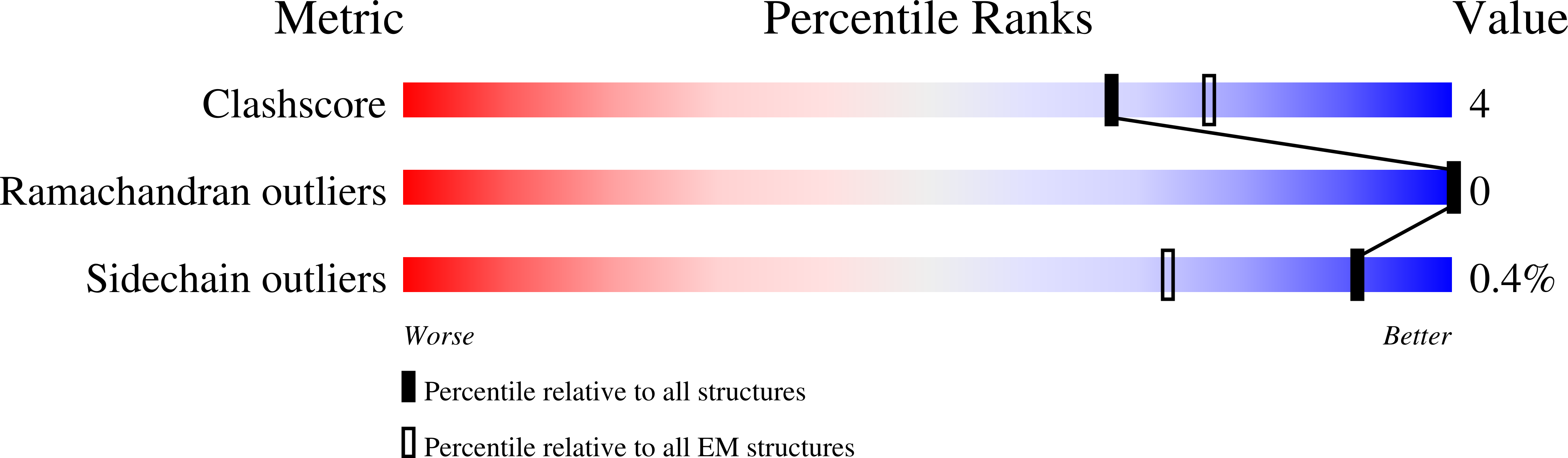Molecular Organisation of Tick-Borne Encephalitis Virus.
Pulkkinen, L.I.A., Barrass, S.V., Domanska, A., Overby, A.K., Anastasina, M., Butcher, S.J.(2022) Viruses 14
- PubMed: 35458522
- DOI: https://doi.org/10.3390/v14040792
- Primary Citation of Related Structures:
7Z51 - PubMed Abstract:
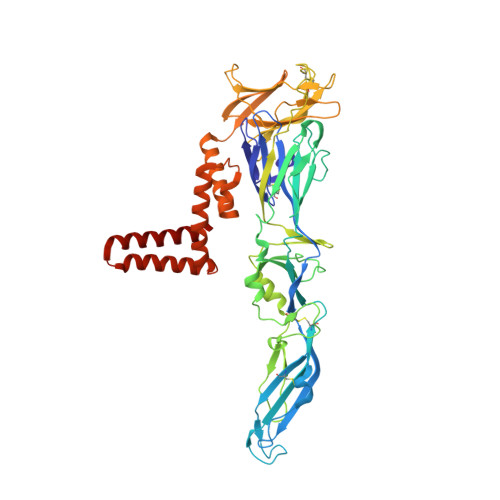
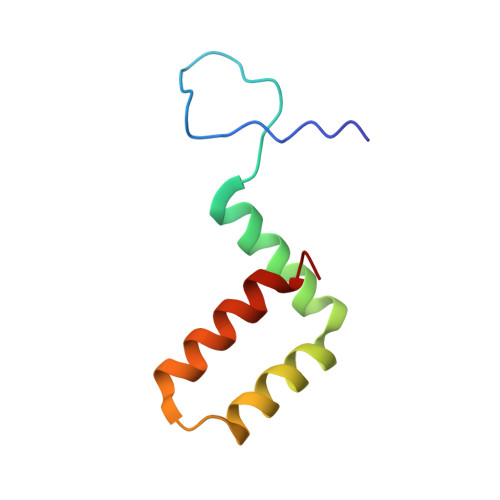
Tick-borne encephalitis virus (TBEV) is a pathogenic, enveloped, positive-stranded RNA virus in the family Flaviviridae . Structural studies of flavivirus virions have primarily focused on mosquito-borne species, with only one cryo-electron microscopy (cryo-EM) structure of a tick-borne species published. Here, we present a 3.3 Å cryo-EM structure of the TBEV virion of the Kuutsalo-14 isolate, confirming the overall organisation of the virus. We observe conformational switching of the peripheral and transmembrane helices of M protein, which can explain the quasi-equivalent packing of the viral proteins and highlights their importance in stabilising membrane protein arrangement in the virion. The residues responsible for M protein interactions are highly conserved in TBEV but not in the structurally studied Hypr strain, nor in mosquito-borne flaviviruses. These interactions may compensate for the lower number of hydrogen bonds between E proteins in TBEV compared to the mosquito-borne flaviviruses. The structure reveals two lipids bound in the E protein which are important for virus assembly. The lipid pockets are comparable to those recently described in mosquito-borne Zika, Spondweni, Dengue, and Usutu viruses. Our results thus advance the understanding of tick-borne flavivirus architecture and virion-stabilising interactions.
Organizational Affiliation:
Faculty of Biological and Environmental Sciences, Molecular and Integrative Bioscience Research Programme, University of Helsinki, 00014 Helsinki, Finland.