Crystal Structure Based Mutagenesis of Cattleyene Synthase Leads to the Generation of Rearranged Polycyclic Diterpenes.
Xing, B., Xu, H., Li, A., Lou, T., Xu, M., Wang, K., Xu, Z., Dickschat, J.S., Yang, D., Ma, M.(2022) Angew Chem Int Ed Engl 61: e202209785-e202209785
- PubMed: 35819825
- DOI: https://doi.org/10.1002/anie.202209785
- Primary Citation of Related Structures:
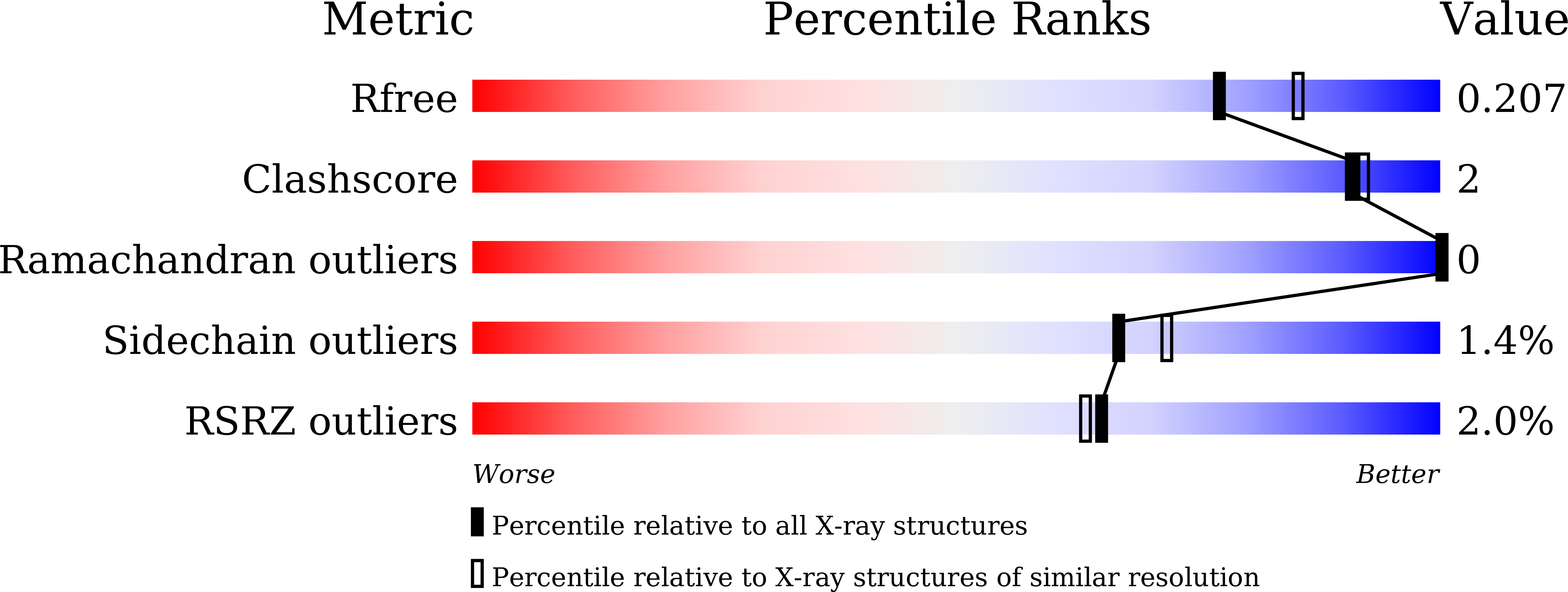
7Y50, 7Y87, 7Y88 - PubMed Abstract:
The crystal structures of cattleyene synthase (apo-CyS), and CyS complexed with geranylgeranyl pyrophosphate (GGPP) were solved. The CyS C59A variant exhibited an increased production of cattleyene and other diterpenes with diverse skeletons. Its structure showed a widened active site cavity explaining the relaxed selectivity. Isotopic labeling experiments revealed a remarkable cyclization mechanism involving several skeletal rearrangements for one of the novel diterpenes.
Organizational Affiliation:
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Haidian District, Beijing, 100191, China.