Structural basis for proteolytic processing of Aspergillus sojae alpha-glucosidase L with strong transglucosylation activity.
Ding, Y., Oyagi, A., Miyasaka, Y., Kozono, T., Sasaki, N., Kojima, Y., Yoshida, M., Matsumoto, Y., Yasutake, N., Nishikawa, A., Tonozuka, T.(2022) J Struct Biol 214: 107874-107874
- PubMed: 35688347
- DOI: https://doi.org/10.1016/j.jsb.2022.107874
- Primary Citation of Related Structures:
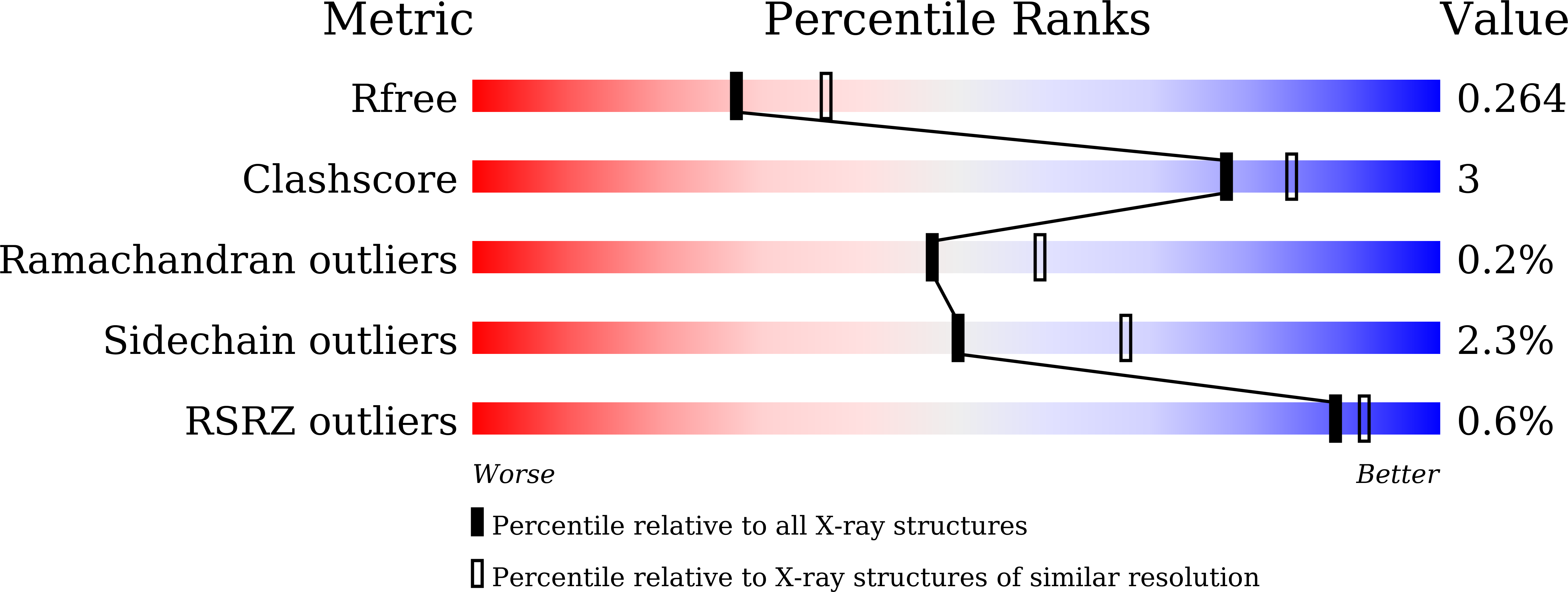
7XOI - PubMed Abstract:
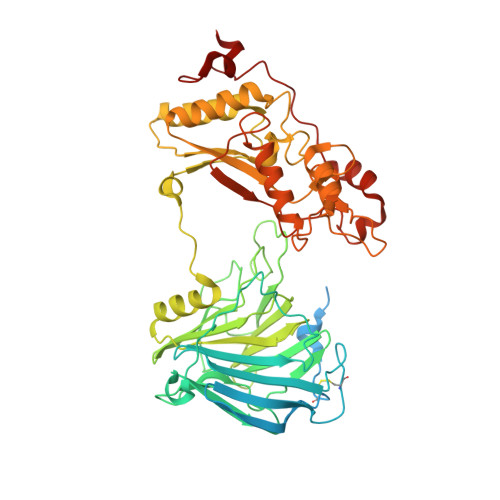
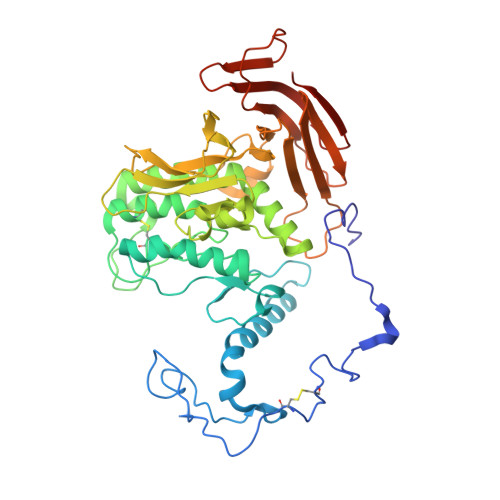
An α-glucosidase from Aspergillus sojae, AsojAgdL, exhibits strong transglucosylation activity to produce α-1,6-glucosidic linkages. The most remarkable structural feature of AsojAgdL is that residues 457-560 of AsojAgdL (designated the NC sequence) is not conserved in other glycoside hydrolase family 31 enzymes, and part of this NC sequence is proteolytically cleaved during its maturation. In this study, the enzyme was expressed in Pichia pastoris, and electrophoretic analysis indicated that the recombinant enzyme, rAsojAgdL, consisted of two polypeptide chains, as observed in the case of the enzyme produced in an Aspergillus strain. The crystal structure of rAsojAgdL was determined in complex with the substrate analog trehalose. Electron density corresponding to residues 496-515 of the NC sequence was not seen, and there were no α-helices or β-strands except for a short α-helix in the structures of residues 457-495 and residues 516-560, both of which belong to the NC sequence. The residues 457-495 and the residues 516-560 both formed extra components of the catalytic domain. The residues 457-495 constituted the entrance of the catalytic pocket of rAsojAgdL, and Gly467, Asp468, Pro469, and Pro470 in the NC sequence were located within 4 Å of Trp400, a key residue involved in binding of the substrate. The results suggest that the proteolytic processing of the NC sequence is related to the formation of the catalytic pocket of AsojAgdL.
Organizational Affiliation:
Department of Applied Biological Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan.