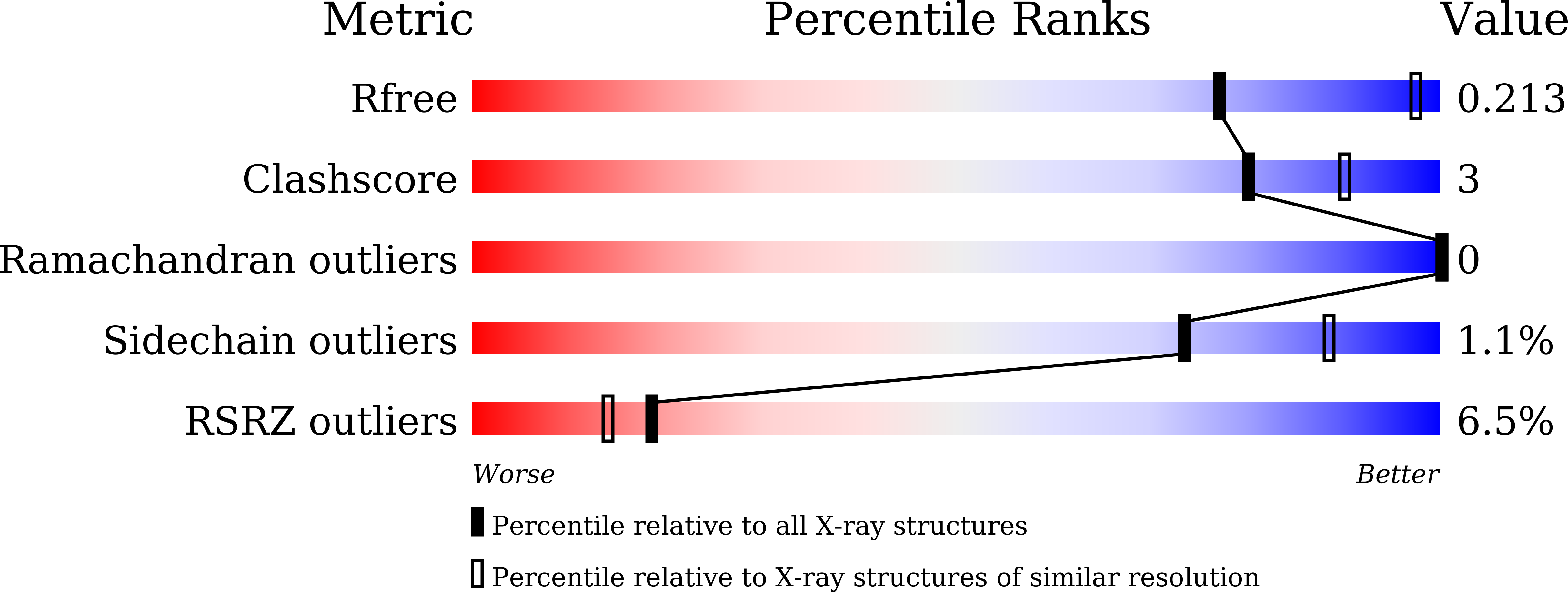
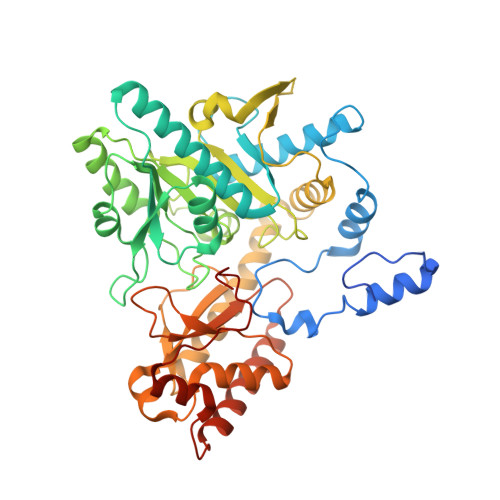
Coordinated regulation of Bacteroides thetaiotaomicron glutamate decarboxylase activity by multiple elements under different pH.
Liu, S., Wen, B., Du, G., Wang, Y., Ma, X., Yu, H., Zhang, J., Fan, S., Zhou, H., Xin, F.(2023) Food Chem 403: 134436-134436
- PubMed: 36358099
- DOI: https://doi.org/10.1016/j.foodchem.2022.134436
- Primary Citation of Related Structures:
7X4L, 7X4Y, 7X51, 7X52 - PubMed Abstract:
Glutamate decarboxylase catalyzes the conversion of glutamate to γ-aminobutyric acid, which plays a vital role in the gut-brain axis. Herein, a novel glutamate decarboxylase from Bacteroides thetaiotaomicron (BTGAD) was heterologously expressed. BTGAD possessed high catalytic efficiency at 60℃ and pH 3.6. As pH response, N-terminal sequence (NTS), C-terminal sequence (CTS), and β-hairpin in BTGAD coordinately regulated its activity under different pH. NTS folded into a loop under acidic pH, and the truncation of NTS severely reduced its activity to 4.2%. While CTS occupied the active site under neutral pH and became disordered to release the inhibition effect under acidic conditions. The β-hairpin, located near the active site, swung and formed open and closed conformations, which acted as an activity switch. This study provides the molecular basis for the coordinated regulation mechanism of BTGAD and lays a theoretical foundation for understanding the metabolism of dietary glutamate and its interaction relationships with the gut-brain axis.
Organizational Affiliation:
Laboratory of Biomanufacturing and Food Engineering, Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing 100193, China.