Difference in the Inhibitory Effect of Thiol Compounds and Demetallation Rates from the Zn(II) Active Site of Metallo-beta-lactamases (IMP-1 and IMP-6) Associated with a Single Amino Acid Substitution.
Yamaguchi, Y., Kato, K., Ichimaru, Y., Uenosono, Y., Tawara, S., Ito, R., Matsuse, N., Wachino, J.I., Toma-Fukai, S., Jin, W., Arakawa, Y., Otsuka, M., Fujita, M., Fukuishi, N., Sugiura, K., Imai, M., Kurosaki, H.(2023) ACS Infect Dis 9: 65-78
- PubMed: 36519431
- DOI: https://doi.org/10.1021/acsinfecdis.2c00395
- Primary Citation of Related Structures:
7WZU - PubMed Abstract:
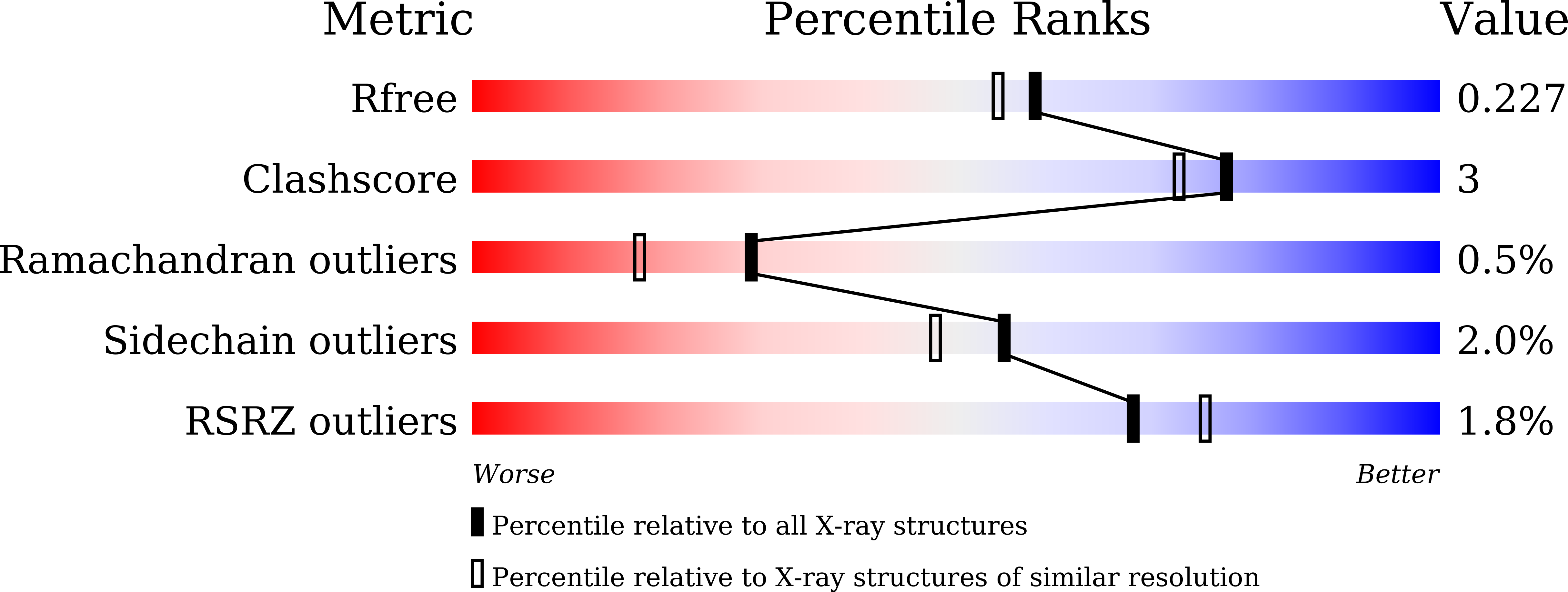
Gram-negative bacteria producing metallo-β-lactamases (MBLs) have become a considerable threat to public health. MBLs including the IMP, VIM, and NDM types are Zn(II) enzymes that hydrolyze the β-lactam ring present in a broad range of antibiotics, such as N -benzylpenicillin, meropenem, and imipenem. Among IMPs, IMP-1 and IMP-6 differ in a single amino acid substitution at position 262, where serine in IMP-1 is replaced by glycine in IMP-6, conferring a change in substrate specificity. To investigate how this mutation influences enzyme function, we examined lactamase inhibition by thiol compounds. Ethyl 3-mercaptopropionate acted as a competitive inhibitor of IMP-1, but a noncompetitive inhibitor of IMP-6. A comparison of the crystal structures previously reported for IMP-1 (PDB code: 5EV6) and IMP-6 (PDB code: 6LVJ) revealed a hydrogen bond between the side chain of Ser262 and Cys221 in IMP-1 but the absence of hydrogen bond in IMP-6, which affects the Zn2 coordination sphere in its active site. We investigated the demetallation rates of IMP-1 and IMP-6 in the presence of chelating agent ethylenediaminetetraacetic acid (EDTA) and found that the demetallation reactions had fast and slow phases with a first-order rate constant ( k fast = 1.76 h -1 , k slow = 0.108 h -1 for IMP-1, and k fast = 14.0 h -1 and k slow = 1.66 h -1 for IMP-6). The difference in the flexibility of the Zn2 coordination sphere between IMP-1 and IMP-6 may influence the demetallation rate, the catalytic efficiency against β-lactam antibiotics, and the inhibitory effect of thiol compounds.
Organizational Affiliation:
Environmental Safety Center, Kumamoto University, 39-1 Kurokami 2-Chome, Chuo-ku, Kumamoto860-8555, Japan.