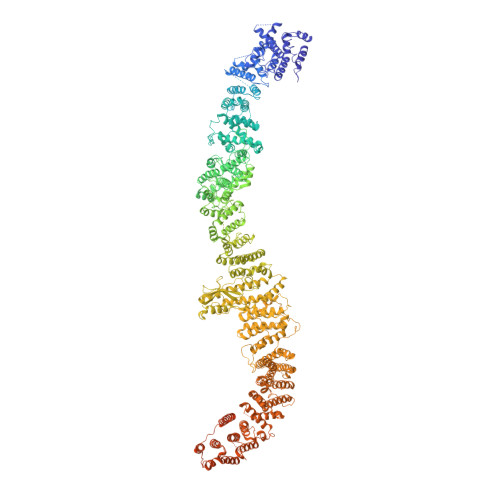
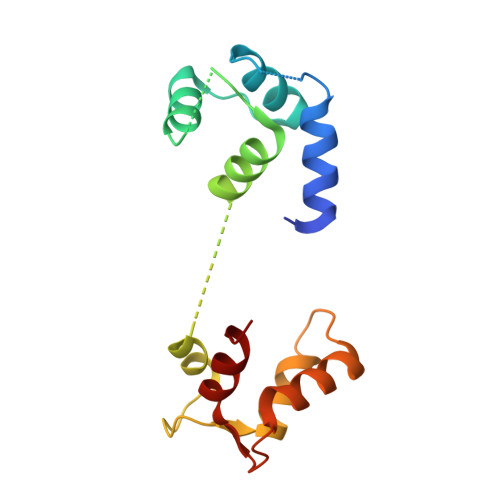
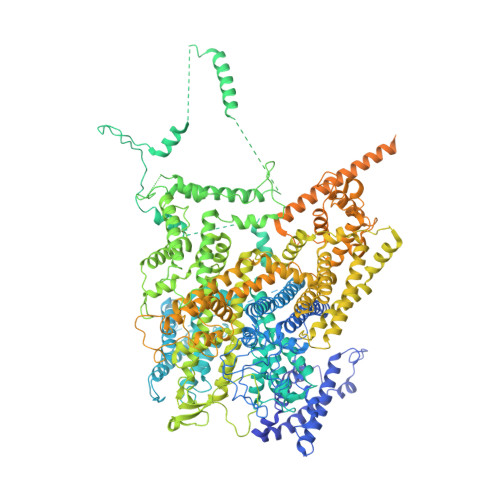
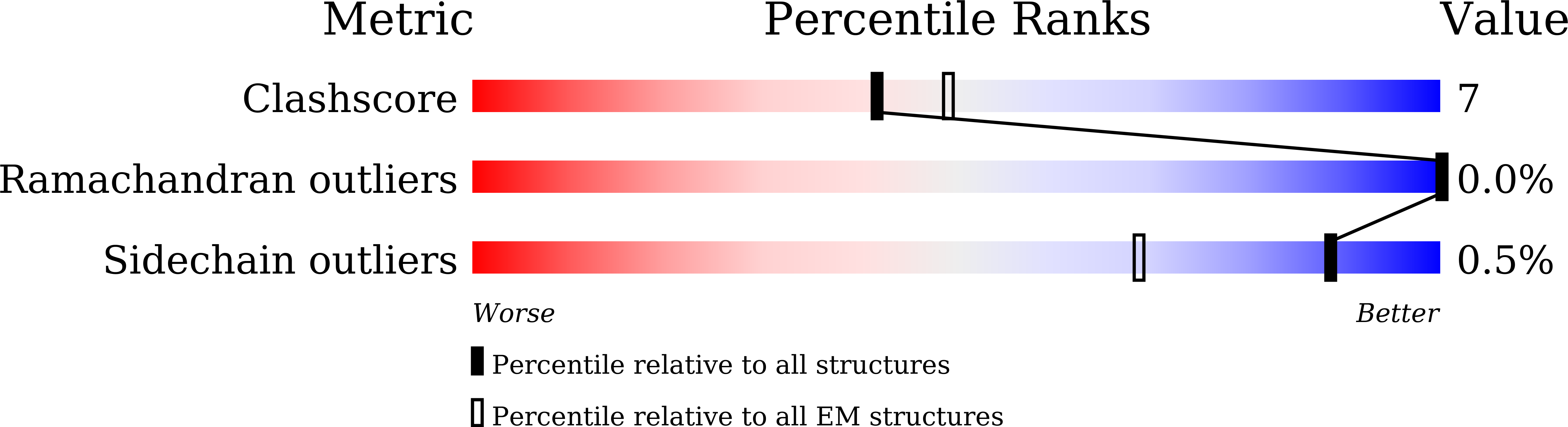Architecture of the human NALCN channelosome.
Zhou, L., Liu, H., Zhao, Q., Wu, J., Yan, Z.(2022) Cell Discov 8: 33-33
- PubMed: 35387979
- DOI: https://doi.org/10.1038/s41421-022-00392-4
- Primary Citation of Related Structures:
7WJI - PubMed Abstract:
NALCN regulates the resting membrane potential by mediating the Na + leak current in neurons, and it functions as a channelosome in complex with FAM155A, UNC79, and UNC80. Dysfunction of the NALCN channelosome causes a broad range of neurological and developmental diseases called NALCN channelopathies in humans. How the auxiliary subunits, especially the two large components UNC79 and UNC80, assemble with NALCN and regulate its function remains unclear. Here we report an overall architecture of the human NALCN channelosome. UNC79 and UNC80 each adopt an S-shape super-helical structure consisting of HEAT and armadillo repeats, forming a super-coiled heterodimeric assembly in the cytoplasmic side, which may provide a scaffold for the binding of other potential modulators of the channelosome. The UNC79-UNC80 assembly specifically associates with the NALCN-FAM155A subcomplex through the intracellular II-III linker of NALCN. Disruptions of the interaction interfaces between UNC79 and UNC80, and between the II-III linker of NALCN and the UNC79-UNC80 assembly, significantly reduce the NALCN-mediated currents in HEK293T system, suggesting the importance of the UNC79-UNC80 assembly in regulating channelosome function. Cross-linking mass spectrometry analysis identified an additional calmodulin (CaM) bound in the carboxyl-terminal domain of NALCN. Our study thus provides a structural basis for understanding the unique assembly mechanism and functional regulation of the NALCN channelosome, and also provides an opportunity for the interpretation of many disease-related mutations in UNC80.
Organizational Affiliation:
Fudan University, Shanghai, China.