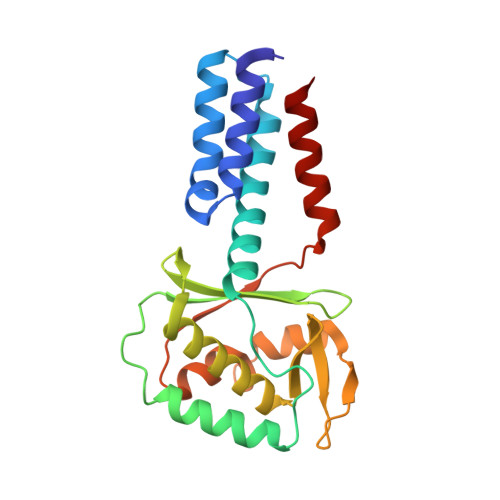
Structural study of Legionella pneumophila effector DotY (Lpg0294), a component of the Dot/Icm type IV secretion system.
Chung, I.Y.W., Cygler, M.(2022) Acta Crystallogr F Struct Biol Commun 78: 276-280
- PubMed: 35787555
- DOI: https://doi.org/10.1107/S2053230X22006604
- Primary Citation of Related Structures:
7UPS - PubMed Abstract:
The bacterium Legionella pneumophila is a causative agent of Legionnaires' disease. It utilizes the Dot/Icm type IV secretion system (T4SS) to deliver over 300 effector proteins into the host cell, leading to modification of cellular processes and creating a safe environment for bacterial proliferation. Dot/Icm is a multi-subunit molecular machine. The effectors are recognized by the inner membrane-embedded coupling complex (T4CC), which then delivers them to the translocation apparatus. This T4CC subcomplex is made up of DotL, DotM, DotN, IcmS, IcmW, LvgA, DotY and DotZ, and its structure was recently determined by cryo-EM. DotY is a highly mobile component of this subcomplex and its structure was only partially defined. DotY is a unique component of the T4SS that is only found in the Legionella genus. Here, the crystal structure of DotY on its own is presented and its fold and the connectivity of its secondary-structure elements are established. The protein is divided into three segments. The first and last segments form a four-helix bundle domain, while the middle segment forms an α/β domain that has a unique fold. The flexibility of the interdomain linkers allows the reorientation of the two domains between that observed in the crystal structure and that assumed within the T4CC subcomplex.
Organizational Affiliation:
Department of Biochemistry, Microbiology and Immunology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.