Defining molecular glues with a dual-nanobody cannabidiol sensor.
Cao, S., Kang, S., Mao, H., Yao, J., Gu, L., Zheng, N.(2022) Nat Commun 13: 815-815
- PubMed: 35145136
- DOI: https://doi.org/10.1038/s41467-022-28507-1
- Primary Citation of Related Structures:
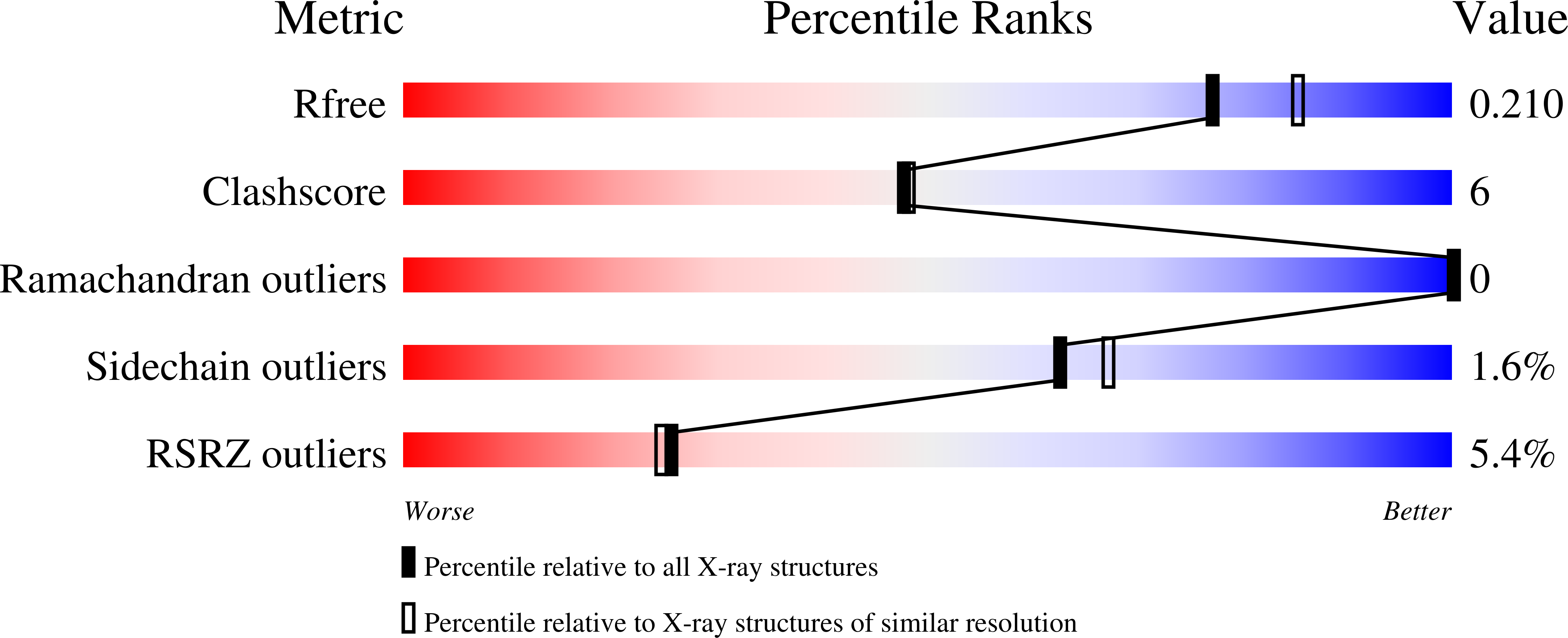
7TE8 - PubMed Abstract:
"Molecular glue" (MG) is a term coined to describe the mechanism of action of the plant hormone auxin and subsequently used to characterize synthetic small molecule protein degraders exemplified by immune-modulatory imide drugs (IMiDs). Prospective development of MGs, however, has been hampered by its elusive definition and thermodynamic characteristics. Here, we report the crystal structure of a dual-nanobody cannabidiol-sensing system, in which the ligand promotes protein-protein interaction in a manner analogous to auxin. Through quantitative analyses, we draw close parallels among the dual-nanobody cannabidiol sensor, the auxin perception complex, and the IMiDs-bound CRL4 CRBN E3, which can bind and ubiquitinate "neo-substrates". All three systems, including the recruitment of IKZF1 and CK1α to CRBN, are characterized by the lack of ligand binding activity in at least one protein partner and an under-appreciated preexisting low micromolar affinity between the two proteinaceous subunits that is enhanced by the ligand to reach the nanomolar range. These two unifying features define MGs as a special class of proximity inducers distinct from bifunctional compounds and can be used as criteria to guide target selection for future rational discovery of MGs.
Organizational Affiliation:
Howard Hughes Medical Institute, Department of Pharmacology, University of Washington, Seattle, WA, 98195, USA.