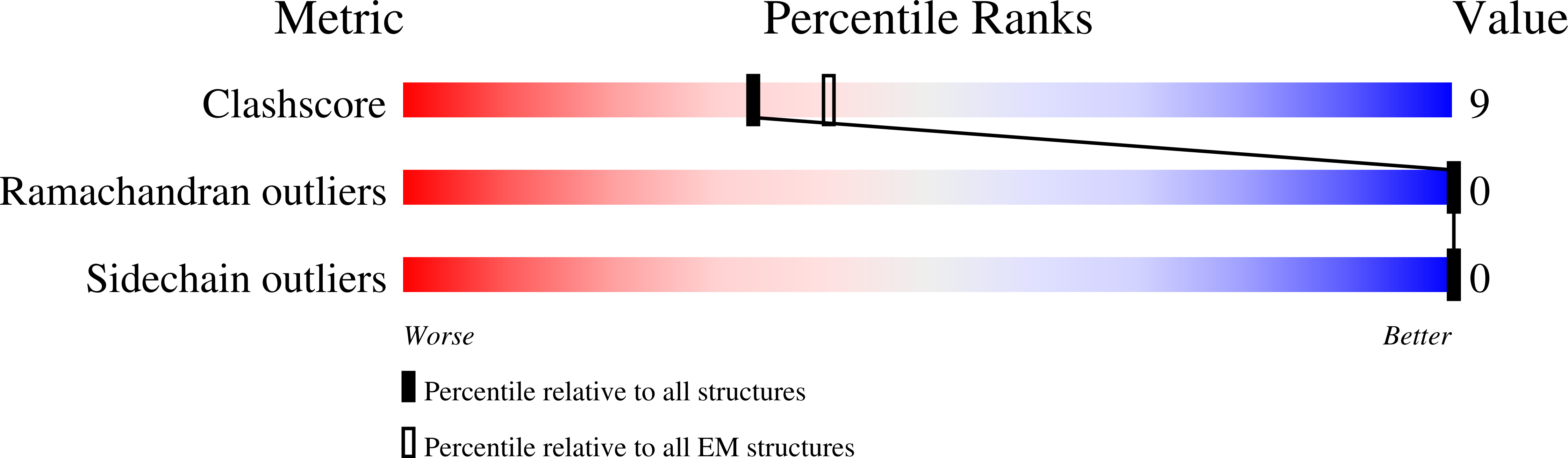Pretransition state and apo structures of the filament-forming enzyme SgrAI elucidate mechanisms of activation and substrate specificity.
Shan, Z., Ghadirian, N., Lyumkis, D., Horton, N.C.(2022) J Biol Chem 298: 101760-101760
- PubMed: 35202658
- DOI: https://doi.org/10.1016/j.jbc.2022.101760
- Primary Citation of Related Structures:
7S8D, 7SS5 - PubMed Abstract:
Enzyme filamentation is a widespread phenomenon that mediates enzyme regulation and function. For the filament-forming sequence-specific DNA endonuclease SgrAI, the process of filamentation both accelerates its DNA cleavage activity and expands its DNA sequence specificity, thus allowing for many additional DNA sequences to be rapidly cleaved. Both outcomes-the acceleration of DNA cleavage and the expansion of sequence specificity-are proposed to regulate critical processes in bacterial innate immunity. However, the mechanistic bases underlying these events remain unclear. Herein, we describe two new structures of the SgrAI enzyme that shed light on its catalytic function. First, we present the cryo-EM structure of filamentous SgrAI bound to intact primary site DNA and Ca 2+ resolved to ∼2.5 Å within the catalytic center, which represents the trapped enzyme-DNA complex prior to the DNA cleavage reaction. This structure reveals important conformational changes that contribute to the catalytic mechanism and the binding of a second divalent cation in the enzyme active site, which is expected to contribute to increased DNA cleavage activity of SgrAI in the filamentous state. Second, we present an X-ray crystal structure of DNA-free (apo) SgrAI resolved to 2.0 Å resolution, which reveals a disordered loop involved in DNA recognition. Collectively, these multiple new observations clarify the mechanism of expansion of DNA sequence specificity of SgrAI, including the indirect readout of sequence-dependent DNA structure, changes in protein-DNA interactions, and the disorder-to-order transition of a crucial DNA recognition element.
Organizational Affiliation:
Laboratory of Genetics, The Salk Institute of Biological Sciences, La Jolla, California, USA.