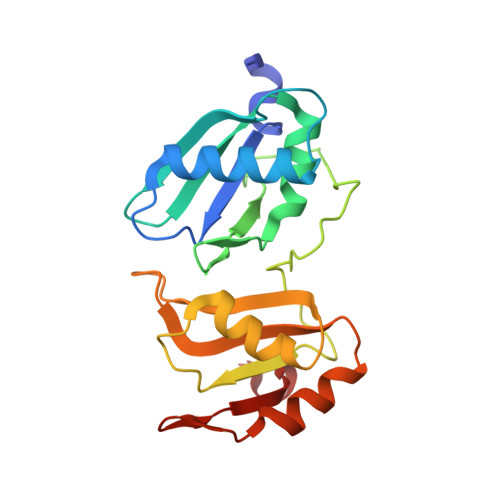
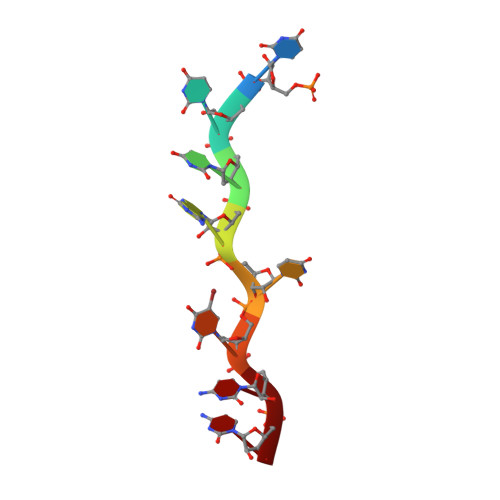
Pre-mRNA splicing factor U2AF2 recognizes distinct conformations of nucleotide variants at the center of the pre-mRNA splice site signal.
Glasser, E., Maji, D., Biancon, G., Puthenpeedikakkal, A.M.K., Cavender, C.E., Tebaldi, T., Jenkins, J.L., Mathews, D.H., Halene, S., Kielkopf, C.L.(2022) Nucleic Acids Res 50: 5299-5312
- PubMed: 35524551
- DOI: https://doi.org/10.1093/nar/gkac287
- Primary Citation of Related Structures:
7S3A, 7S3B, 7S3C - PubMed Abstract:
The essential pre-mRNA splicing factor U2AF2 (also called U2AF65) identifies polypyrimidine (Py) tract signals of nascent transcripts, despite length and sequence variations. Previous studies have shown that the U2AF2 RNA recognition motifs (RRM1 and RRM2) preferentially bind uridine-rich RNAs. Nonetheless, the specificity of the RRM1/RRM2 interface for the central Py tract nucleotide has yet to be investigated. We addressed this question by determining crystal structures of U2AF2 bound to a cytidine, guanosine, or adenosine at the central position of the Py tract, and compared U2AF2-bound uridine structures. Local movements of the RNA site accommodated the different nucleotides, whereas the polypeptide backbone remained similar among the structures. Accordingly, molecular dynamics simulations revealed flexible conformations of the central, U2AF2-bound nucleotide. The RNA binding affinities and splicing efficiencies of structure-guided mutants demonstrated that U2AF2 tolerates nucleotide substitutions at the central position of the Py tract. Moreover, enhanced UV-crosslinking and immunoprecipitation of endogenous U2AF2 in human erythroleukemia cells showed uridine-sensitive binding sites, with lower sequence conservation at the central nucleotide positions of otherwise uridine-rich, U2AF2-bound splice sites. Altogether, these results highlight the importance of RNA flexibility for protein recognition and take a step towards relating splice site motifs to pre-mRNA splicing efficiencies.
Organizational Affiliation:
Department of Biochemistry and Biophysics, and the Center for RNA Biology, University of Rochester School of Medicine and Dentistry, Rochester, NY 14642, USA.