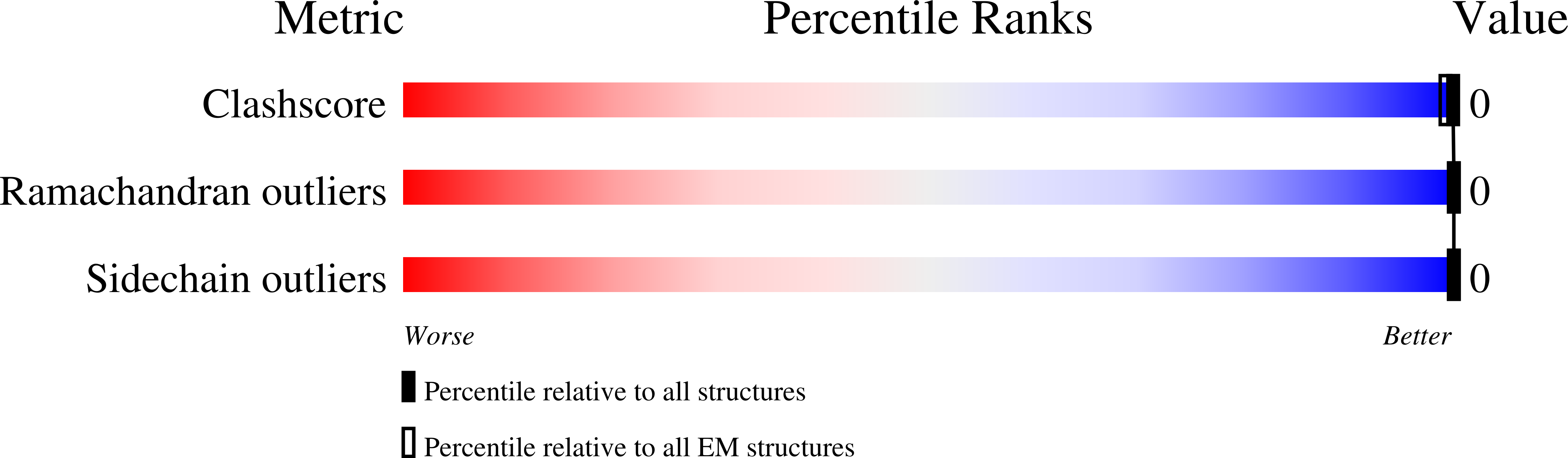Cryo-EM of Helical Polymers.
Wang, F., Gnewou, O., Solemanifar, A., Conticello, V.P., Egelman, E.H.(2022) Chem Rev 122: 14055-14065
- PubMed: 35133794
- DOI: https://doi.org/10.1021/acs.chemrev.1c00753
- Primary Citation of Related Structures:
7RX4, 7RX5 - PubMed Abstract:
While the application of cryogenic electron microscopy (cryo-EM) to helical polymers in biology has a long history, due to the huge number of helical macromolecular assemblies in viruses, bacteria, archaea, and eukaryotes, the use of cryo-EM to study synthetic soft matter noncovalent polymers has been much more limited. This has mainly been due to the lack of familiarity with cryo-EM in the materials science and chemistry communities, in contrast to the fact that cryo-EM was developed as a biological technique. Nevertheless, the relatively few structures of self-assembled peptide nanotubes and ribbons solved at near-atomic resolution by cryo-EM have demonstrated that cryo-EM should be the method of choice for a structural analysis of synthetic helical filaments. In addition, cryo-EM has also demonstrated that the self-assembly of soft matter polymers has enormous potential for polymorphism, something that may be obscured by techniques such as scattering and spectroscopy. These cryo-EM structures have revealed how far we currently are from being able to predict the structure of these polymers due to their chaotic self-assembly behavior.
Organizational Affiliation:
Department of Biochemistry and Molecular Genetics, University of Virginia, Charlottesville, Virginia 22908, United States.