Enabling Efficient Folding and High-Resolution Crystallographic Analysis of Bracelet Cyclotides.
Huang, Y.H., Du, Q., Jiang, Z., King, G.J., Collins, B.M., Wang, C.K., Craik, D.J.(2021) Molecules 26
- PubMed: 34577034
- DOI: https://doi.org/10.3390/molecules26185554
- Primary Citation of Related Structures:
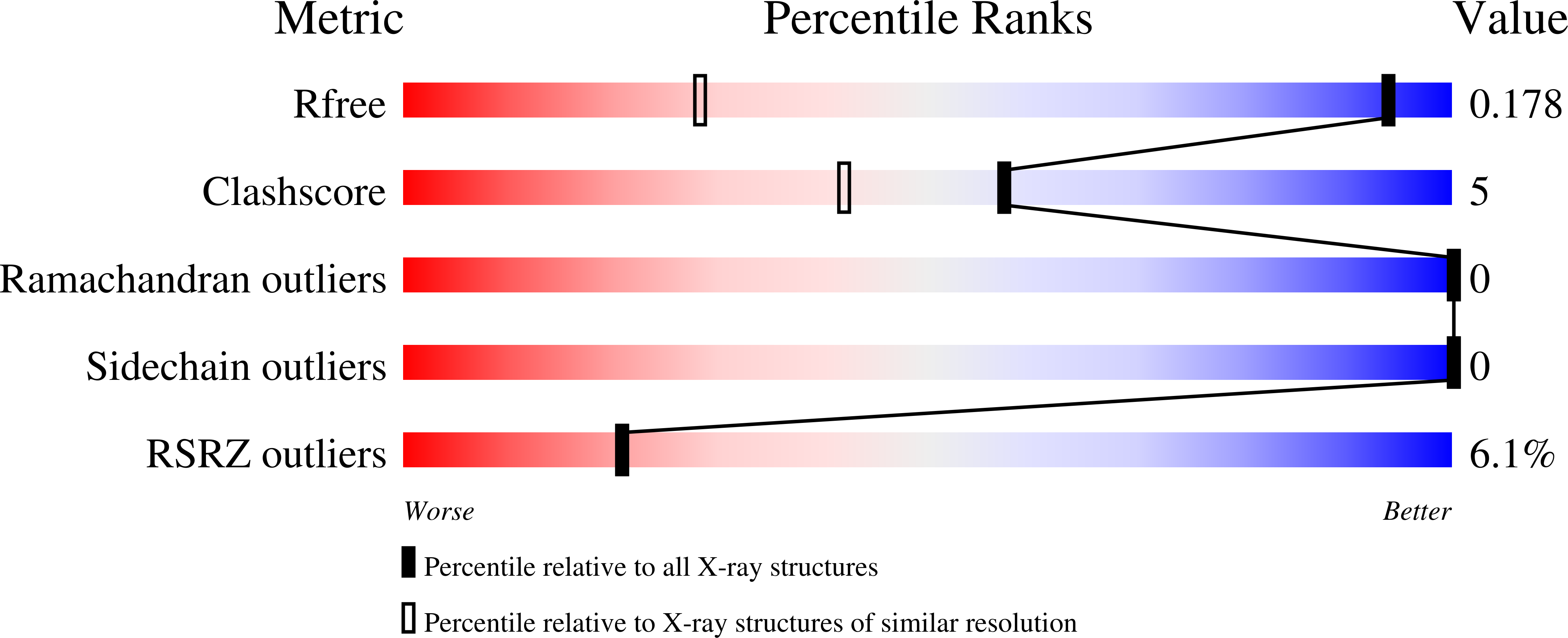
7RMQ, 7RMR, 7RMS - PubMed Abstract:
Cyclotides have attracted great interest as drug design scaffolds because of their unique cyclic cystine knotted topology. They are classified into three subfamilies, among which the bracelet subfamily represents the majority and comprises the most bioactive cyclotides, but are the most poorly utilized in drug design applications. A long-standing challenge has been the very low in vitro folding yields of bracelets, hampering efforts to characterize their structures and activities. Herein, we report substantial increases in bracelet folding yields enabled by a single point mutation of residue Ile-11 to Leu or Gly. We applied this discovery to synthesize mirror image enantiomers and used quasi-racemic crystallography to elucidate the first crystal structures of bracelet cyclotides. This study provides a facile strategy to produce bracelet cyclotides, leading to a general method to easily access their atomic resolution structures and providing a basis for development of biotechnological applications.
Organizational Affiliation:
Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia.