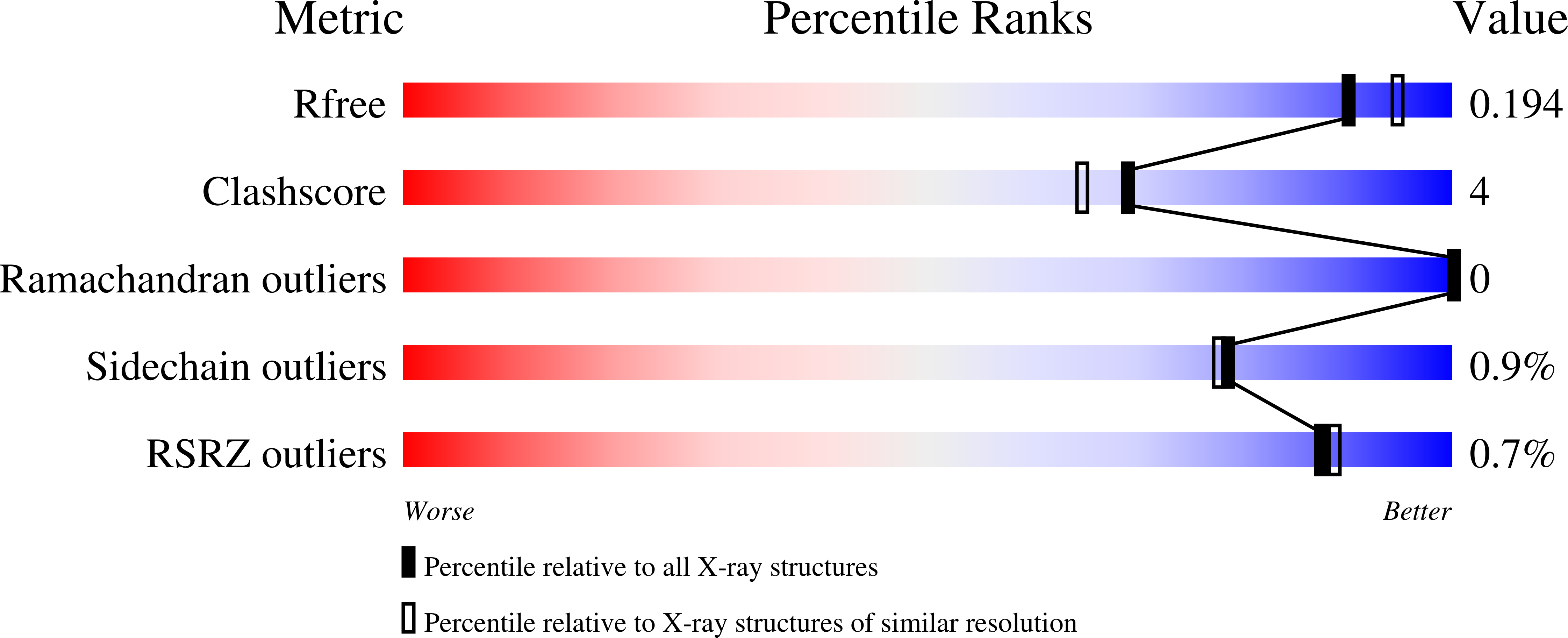
A shared mechanistic pathway for pyridoxal phosphate-dependent arginine oxidases.
Hoffarth, E.R., Caddell Haatveit, K., Kuatsjah, E., MacNeil, G.A., Saroya, S., Walsby, C.J., Eltis, L.D., Houk, K.N., Garcia-Borras, M., Ryan, K.S.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34580201
- DOI: https://doi.org/10.1073/pnas.2012591118
- Primary Citation of Related Structures:
7N79, 7RF9, 7RGB - PubMed Abstract:
The mechanism by which molecular oxygen is activated by the organic cofactor pyridoxal phosphate (PLP) for oxidation reactions remains poorly understood. Recent work has identified arginine oxidases that catalyze desaturation or hydroxylation reactions. Here, we investigate a desaturase from the Pseudoalteromonas luteoviolacea indolmycin pathway. Our work, combining X-ray crystallographic, biochemical, spectroscopic, and computational studies, supports a shared mechanism with arginine hydroxylases, involving two rounds of single-electron transfer to oxygen and superoxide rebound at the 4' carbon of the PLP cofactor. The precise positioning of a water molecule in the active site is proposed to control the final reaction outcome. This proposed mechanism provides a unified framework to understand how oxygen can be activated by PLP-dependent enzymes for oxidation of arginine and elucidates a shared mechanistic pathway and intertwined evolutionary history for arginine desaturases and hydroxylases.
Organizational Affiliation:
Department of Chemistry, The University of British Columbia, Vancouver, BC V6T 1Z1, Canada.