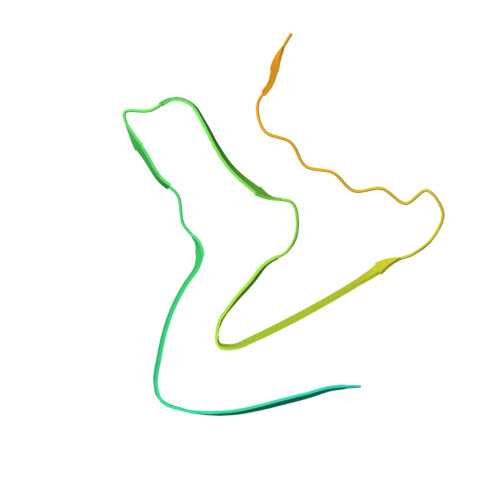
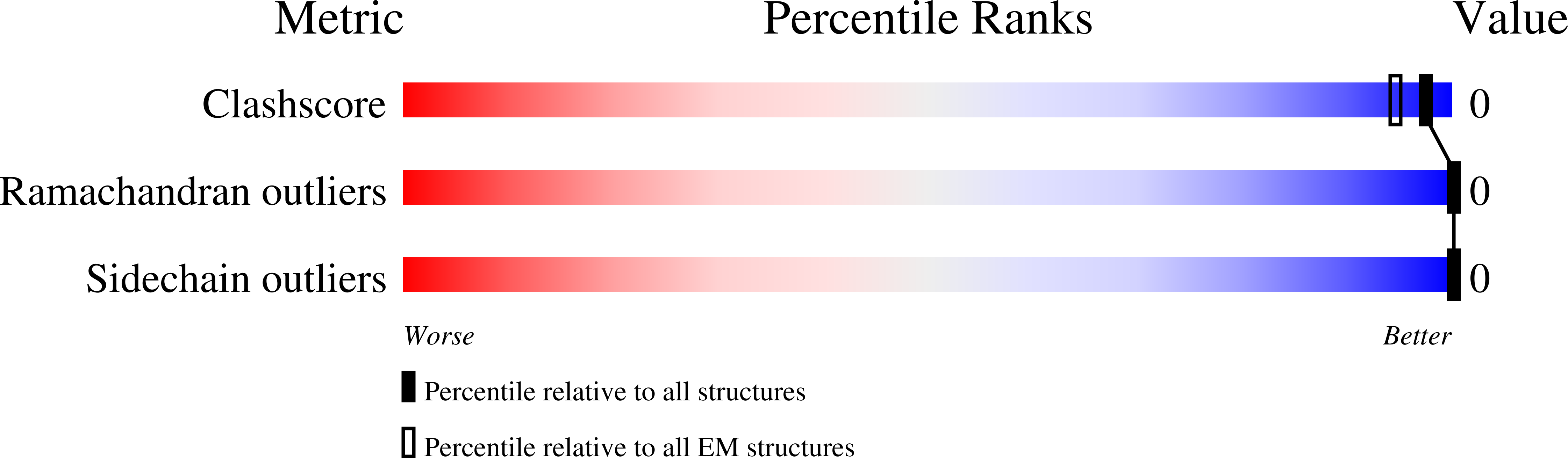Seeded assembly in vitro does not replicate the structures of alpha-synuclein filaments from multiple system atrophy.
Lovestam, S., Schweighauser, M., Matsubara, T., Murayama, S., Tomita, T., Ando, T., Hasegawa, K., Yoshida, M., Tarutani, A., Hasegawa, M., Goedert, M., Scheres, S.H.W.(2021) FEBS Open Bio 11: 999-1013
- PubMed: 33548114
- DOI: https://doi.org/10.1002/2211-5463.13110
- Primary Citation of Related Structures:
7NCA, 7NCG, 7NCH, 7NCI, 7NCJ, 7NCK - PubMed Abstract:
The propagation of conformational strains by templated seeding is central to the prion concept. Seeded assembly of α-synuclein into filaments is believed to underlie the prion-like spreading of protein inclusions in a number of human neurodegenerative diseases, including Parkinson's disease, dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). We previously determined the atomic structures of α-synuclein filaments from the putamen of five individuals with MSA. Here, we used filament preparations from three of these brains for the in vitro seeded assembly of recombinant human α-synuclein. We find that the structures of the seeded assemblies differ from those of the seeds, suggesting that additional, as yet unknown, factors play a role in the propagation of the seeds. Identification of these factors will be essential for understanding the prion-like spreading of α-synuclein proteinopathies.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge, UK.