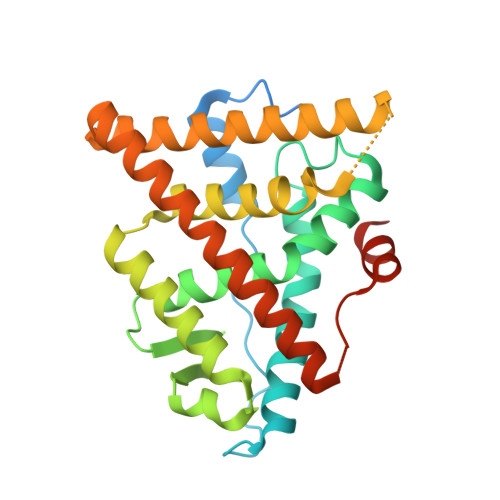
GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer.
Liang, J., Zbieg, J.R., Blake, R.A., Chang, J.H., Daly, S., DiPasquale, A.G., Friedman, L.S., Gelzleichter, T., Gill, M., Giltnane, J.M., Goodacre, S., Guan, J., Hartman, S.J., Ingalla, E.R., Kategaya, L., Kiefer, J.R., Kleinheinz, T., Labadie, S.S., Lai, T., Li, J., Liao, J., Liu, Z., Mody, V., McLean, N., Metcalfe, C., Nannini, M.A., Oeh, J., O'Rourke, M.G., Ortwine, D.F., Ran, Y., Ray, N.C., Roussel, F., Sambrone, A., Sampath, D., Schutt, L.K., Vinogradova, M., Wai, J., Wang, T., Wertz, I.E., White, J.R., Yeap, S.K., Young, A., Zhang, B., Zheng, X., Zhou, W., Zhong, Y., Wang, X.(2021) J Med Chem 64: 11841-11856
- PubMed: 34251202
- DOI: https://doi.org/10.1021/acs.jmedchem.1c00847
- Primary Citation of Related Structures:
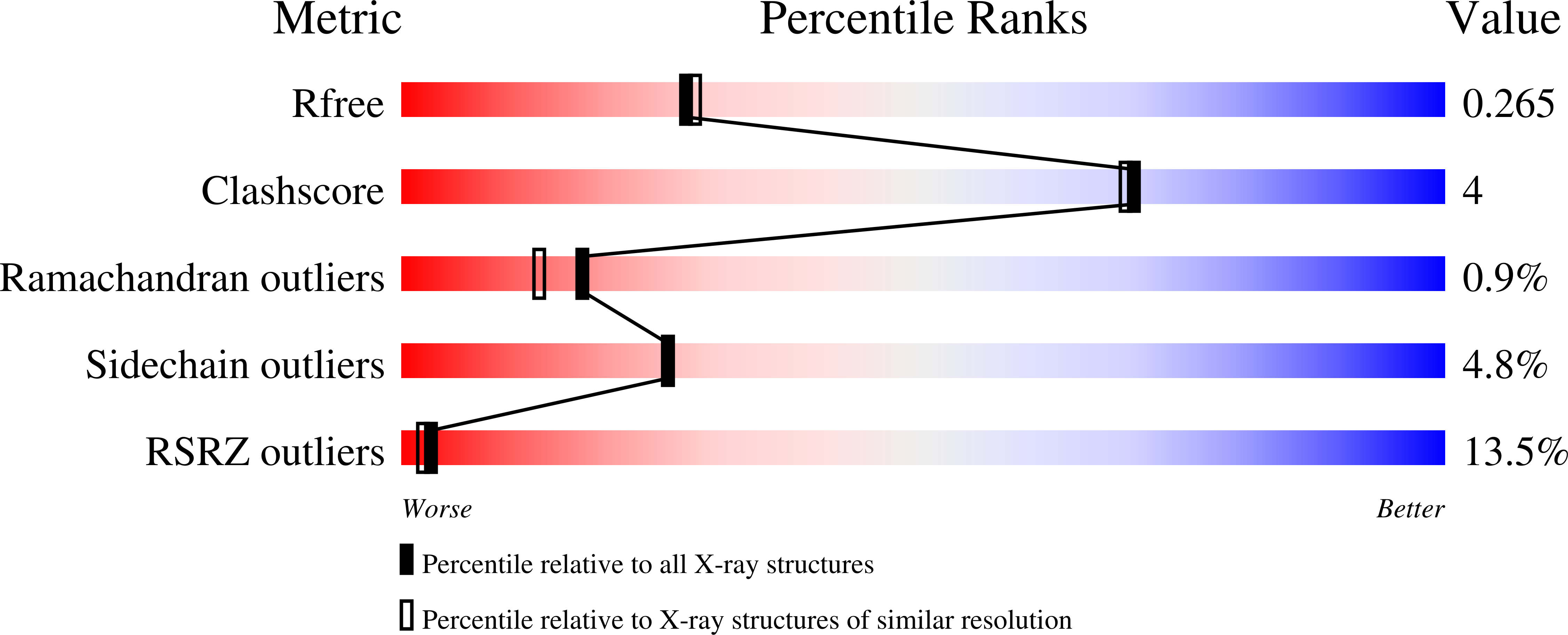
7MSA - PubMed Abstract:
Breast cancer remains a leading cause of cancer death in women, representing a significant unmet medical need. Here, we disclose our discovery efforts culminating in a clinical candidate, 35 (GDC-9545 or giredestrant). 35 is an efficient and potent selective estrogen receptor degrader (SERD) and a full antagonist, which translates into better antiproliferation activity than known SERDs ( 1 , 6 , 7 , and 9 ) across multiple cell lines. Fine-tuning the physiochemical properties enabled once daily oral dosing of 35 in preclinical species and humans. 35 exhibits low drug-drug interaction liability and demonstrates excellent in vitro and in vivo safety profiles. At low doses, 35 induces tumor regressions either as a single agent or in combination with a CDK4/6 inhibitor in an ESR1 Y537S mutant PDX or a wild-type ERα tumor model. Currently, 35 is being evaluated in Phase III clinical trials.
Organizational Affiliation:
Genentech, Inc., 1 DNA Way, South San Francisco, California 94080, United States.