A distributed residue network permits conformational binding specificity in a conserved family of actin remodelers.
Hwang, T., Parker, S.S., Hill, S.M., Ilunga, M.W., Grant, R.A., Mouneimne, G., Keating, A.E.(2021) Elife 10
- PubMed: 34854809
- DOI: https://doi.org/10.7554/eLife.70601
- Primary Citation of Related Structures:
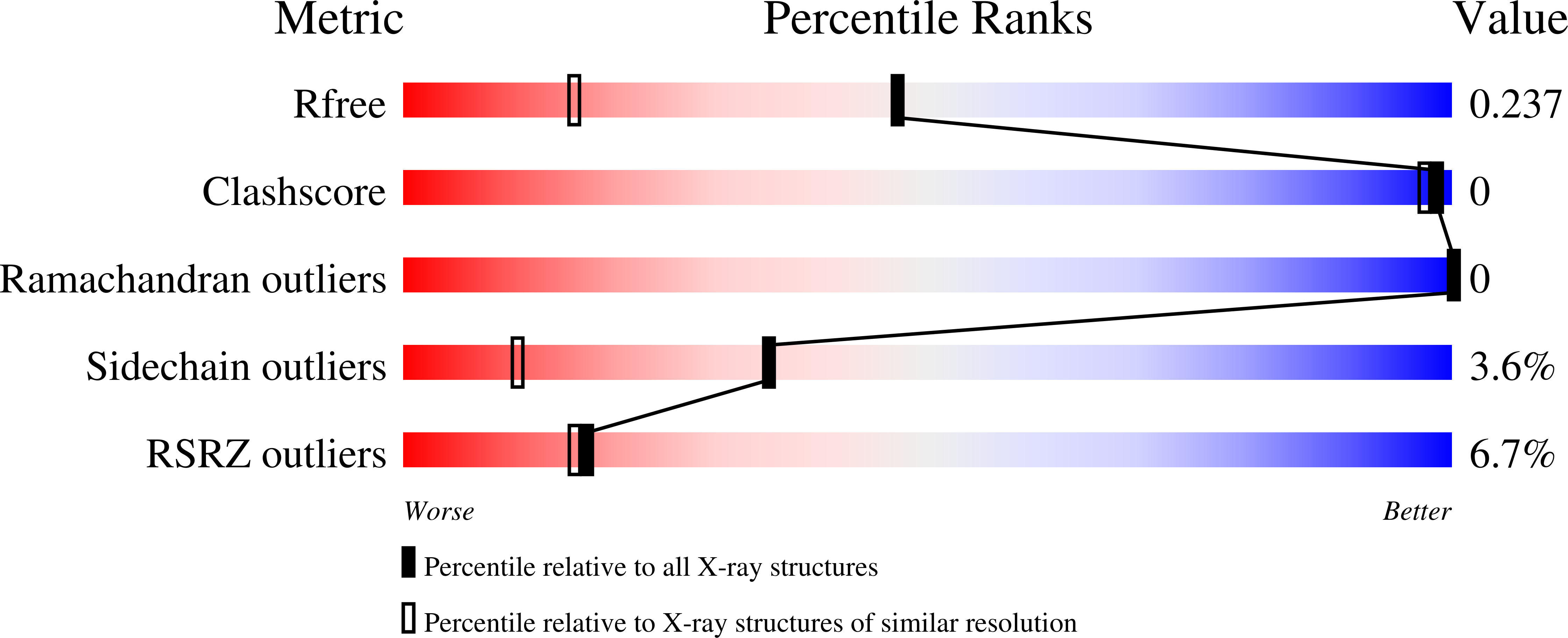
7LXF - PubMed Abstract:
Metazoan proteomes contain many paralogous proteins that have evolved distinct functions. The Ena/VASP family of actin regulators consists of three members that share an EVH1 interaction domain with a 100 % conserved binding site. A proteome-wide screen revealed photoreceptor cilium actin regulator (PCARE) as a high-affinity ligand for ENAH EVH1. Here, we report the surprising observation that PCARE is ~100-fold specific for ENAH over paralogs VASP and EVL and can selectively bind ENAH and inhibit ENAH-dependent adhesion in cells. Specificity arises from a mechanism whereby PCARE stabilizes a conformation of the ENAH EVH1 domain that is inaccessible to family members VASP and EVL. Structure-based modeling rapidly identified seven residues distributed throughout EVL that are sufficient to differentiate binding by ENAH vs. EVL. By exploiting the ENAH-specific conformation, we rationally designed the tightest and most selective ENAH binder to date. Our work uncovers a conformational mechanism of interaction specificity that distinguishes highly similar paralogs and establishes tools for dissecting specific Ena/VASP functions in processes including cancer cell invasion.
Organizational Affiliation:
Department of Biology, Massachusetts Institute of Technology, Cambridge, United States.