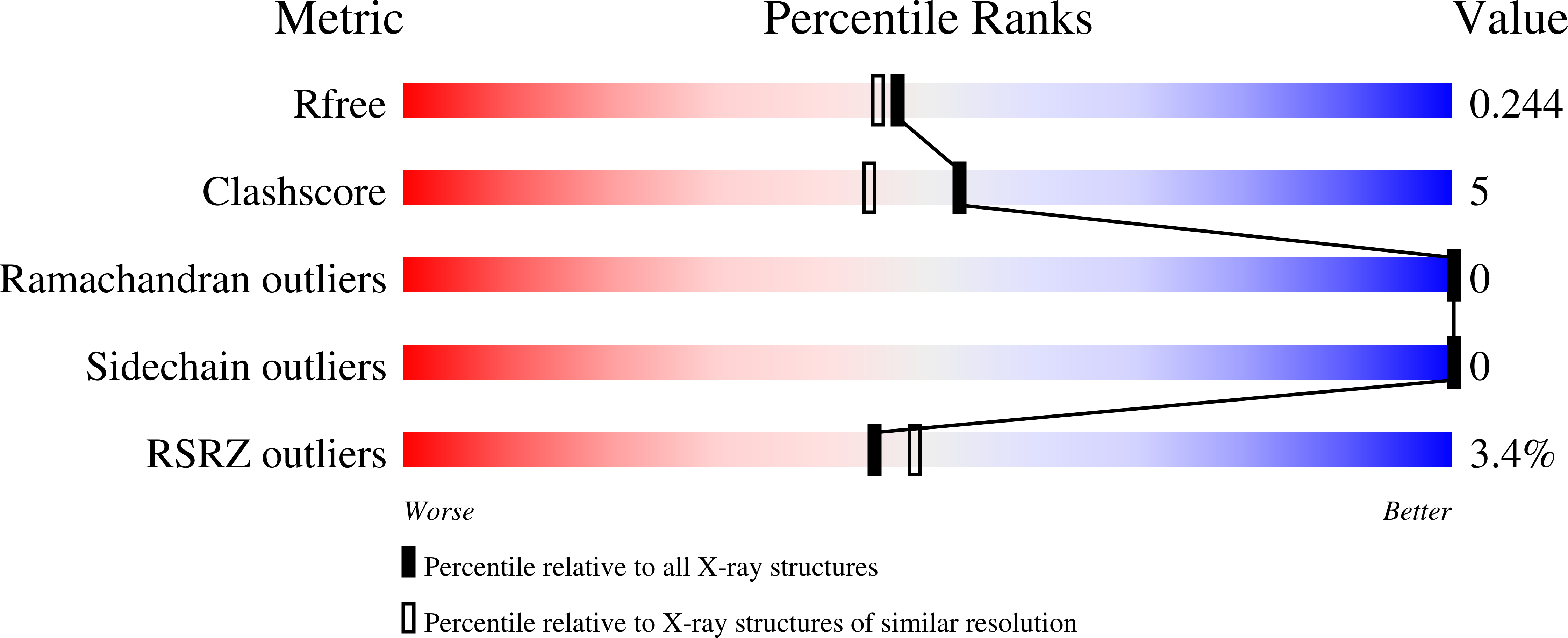
Altered APE1 activity on abasic ribonucleotides is mediated by changes in the nucleoside sugar pucker.
Hoitsma, N.M., Click, T.H., Agarwal, P.K., Freudenthal, B.D.(2021) Comput Struct Biotechnol J 19: 3293-3302
- PubMed: 34188778
- DOI: https://doi.org/10.1016/j.csbj.2021.05.035
- Primary Citation of Related Structures:
7LPG, 7LPH, 7LPI, 7LPJ - PubMed Abstract:
Ribonucleotides (rNTPs) are predicted to be incorporated into the genome at a rate of up to 3 million times per cell division, making rNTPs the most common non-standard nucleotide in the human genome. Typically, misinserted ribonucleotides are repaired by the ribonucleotide excision repair (RER) pathway, which is initiated by RNase H2 cleavage. However, rNTPs are susceptible to spontaneous depurination generating abasic ribonucleotides (rAPs), which are unable to be processed by RNase H2. Additionally, rAPs have been found in nascent RNA and coupled to R-loops. Recent work identified that base excision repair (BER) protein AP-Endonuclease 1 (APE1) is responsible for the initial processing of rAPs embedded in DNA and in R-loops. APE1 is a well characterized AP endonuclease that cleaves 5' of abasic sites, but its ability to cleave at rAPs remains poorly understood. Here, we utilize enzyme kinetics, X-ray crystallography, and molecular dynamics simulations to provide insight into rAP processing by APE1. Enzyme kinetics were used to determine pre-steady-state rates of APE1 cleavage on DNA substrates containing rAP, revealing a decrease in activity compared to cleavage at a canonical deoxy-AP substrate. Using X-ray crystallography, we identified novel contacts between the rAP and the APE1 active site. We demonstrate that the rAP sugar pucker is accommodated in the active site in a C3'-endo conformation, influencing its position and contributing to a decrease in activity compared to the deoxy-AP site. Together, this work provides molecular level insights into rAP processing by APE1 and advances our understanding of ribonucleotide processing within genomic DNA.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Kansas Medical Center, Kansas City, KS 66160, USA.