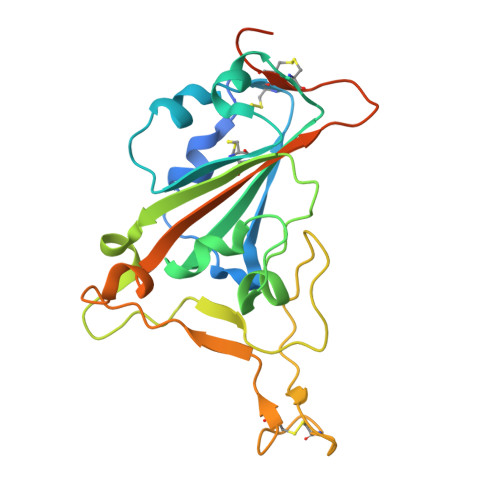
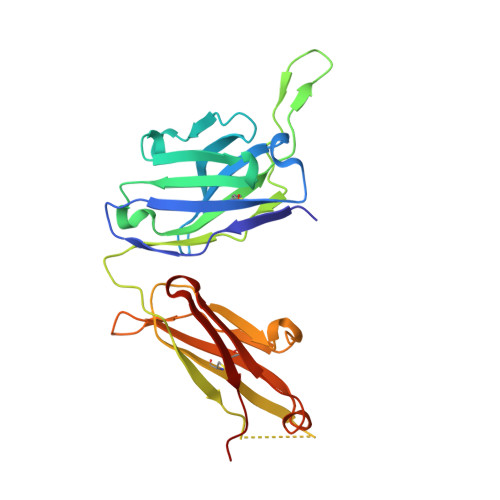
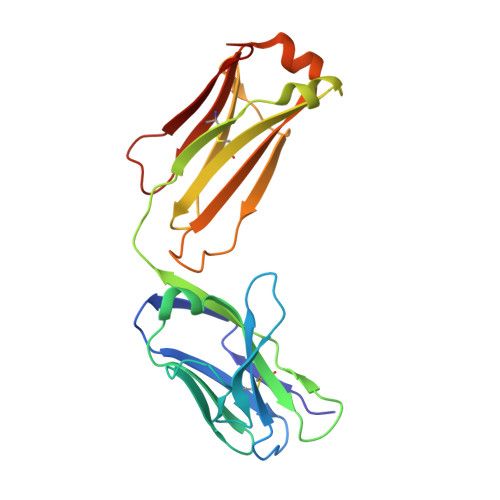
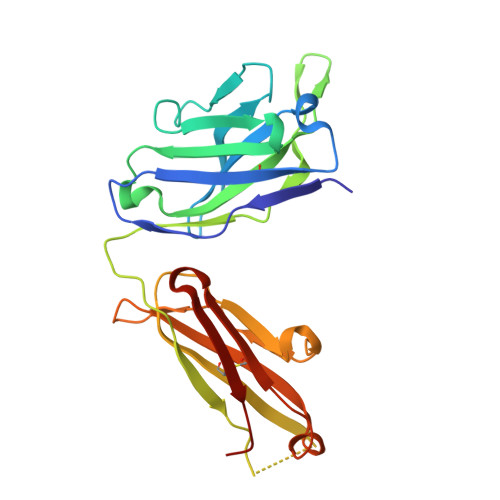
A combination of cross-neutralizing antibodies synergizes to prevent SARS-CoV-2 and SARS-CoV pseudovirus infection.
Liu, H., Yuan, M., Huang, D., Bangaru, S., Zhao, F., Lee, C.D., Peng, L., Barman, S., Zhu, X., Nemazee, D., Burton, D.R., van Gils, M.J., Sanders, R.W., Kornau, H.C., Reincke, S.M., Pruss, H., Kreye, J., Wu, N.C., Ward, A.B., Wilson, I.A.(2021) Cell Host Microbe 29: 806-818.e6
- PubMed: 33894127
- DOI: https://doi.org/10.1016/j.chom.2021.04.005
- Primary Citation of Related Structures:
7LM8, 7LM9 - PubMed Abstract:
Coronaviruses have caused several human epidemics and pandemics including the ongoing coronavirus disease 2019 (COVID-19). Prophylactic vaccines and therapeutic antibodies have already shown striking effectiveness against COVID-19. Nevertheless, concerns remain about antigenic drift in SARS-CoV-2 as well as threats from other sarbecoviruses. Cross-neutralizing antibodies to SARS-related viruses provide opportunities to address such concerns. Here, we report on crystal structures of a cross-neutralizing antibody, CV38-142, in complex with the receptor-binding domains from SARS-CoV-2 and SARS-CoV. Recognition of the N343 glycosylation site and water-mediated interactions facilitate cross-reactivity of CV38-142 to SARS-related viruses, allowing the antibody to accommodate antigenic variation in these viruses. CV38-142 synergizes with other cross-neutralizing antibodies, notably COVA1-16, to enhance neutralization of SARS-CoV and SARS-CoV-2, including circulating variants of concern B.1.1.7 and B.1.351. Overall, this study provides valuable information for vaccine and therapeutic design to address current and future antigenic drift in SARS-CoV-2 and to protect against zoonotic SARS-related coronaviruses.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.