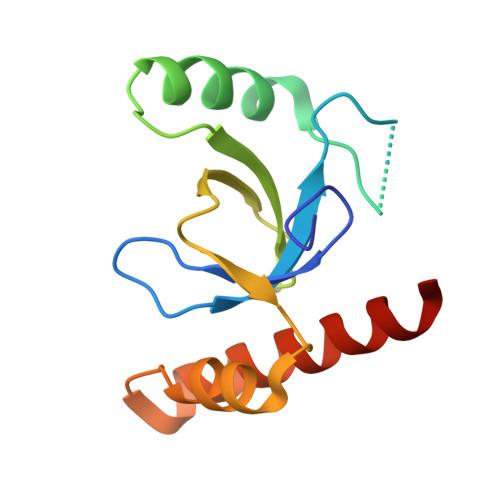
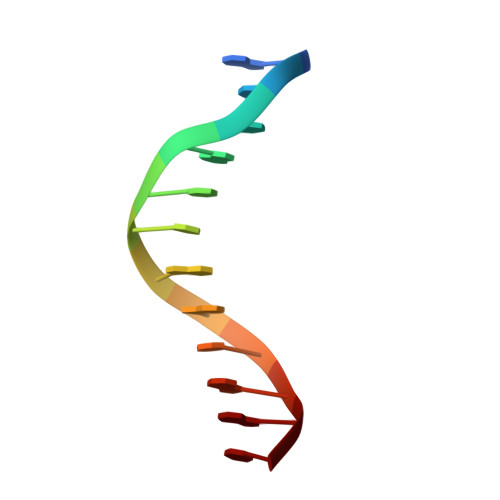
Crystal structure of the BRPF2 PWWP domain in complex with DNA reveals a different binding mode than the HDGF family of PWWP domains.
Zhang, M., Lei, M., Qin, S., Dong, A., Yang, A., Li, Y., Loppnau, P., Hughes, T.R., Min, J., Liu, Y.(2021) Biochim Biophys Acta Gene Regul Mech 1864: 194688-194688
- PubMed: 33556623
- DOI: https://doi.org/10.1016/j.bbagrm.2021.194688
- Primary Citation of Related Structures:
7LH9 - PubMed Abstract:
The PWWP domain was first identified in the HDGF protein family and named after the conserved Proline-Tryptophan-Tryptophan-Proline motif in WHSC1. The PWWP domain-containing proteins play important roles in different biological processes, such as DNA replication, transcription, DNA repair, pre-mRNA processing by recognizing methylated histone and dsDNA simultaneously. Recently, how the HDGF family of PWWP domains recognize histone H3K36me3-modified nucleosome has been reported. In order to better understand the interactions between the PWWP domain and dsDNA, we carried out family-wide characterization of dsDNA binding abilities of human PWWP domains. Our binding assays confirmed that PWWP domains bind to dsDNA without sequence selectivity. Our crystal structure of the BRPF2 PWWP domain in complex with a 12-mer dsDNA reveals that the PWWP domain interacts with dsDNA by binding to its major groove, instead of the minor groove observed in the HDGF family of PWWP domains. Our study indicates that PWWP domains could bind to dsDNA in different modes.
Organizational Affiliation:
College of Pharmaceutical Sciences, Soochow University, Suzhou, Jiangsu, China.