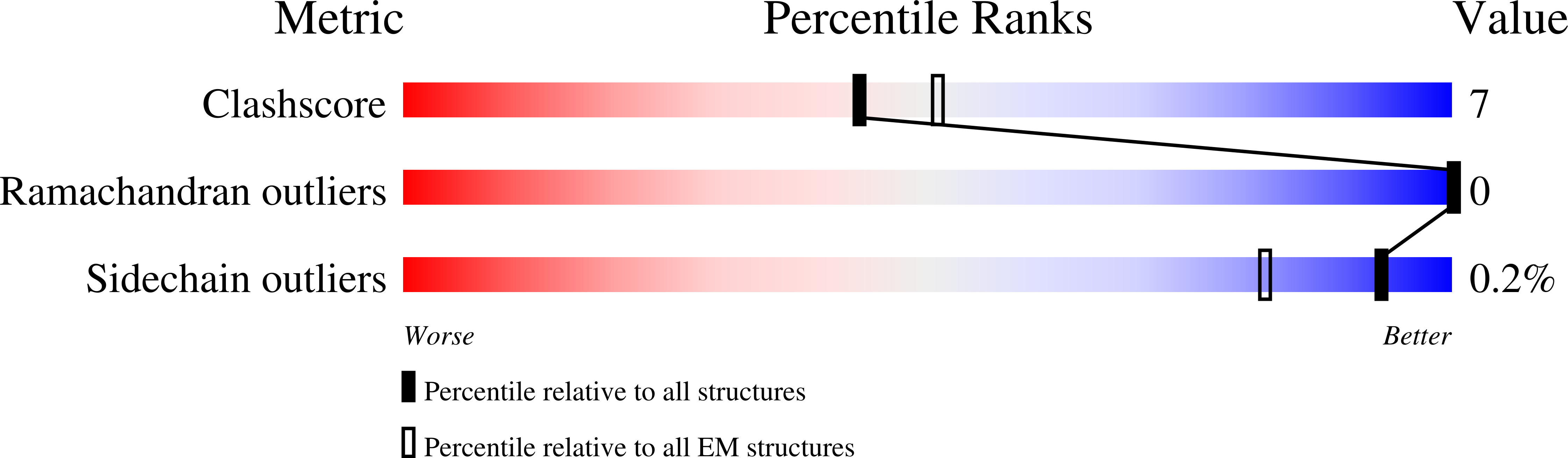Structural basis of TRAPPIII-mediated Rab1 activation.
Joiner, A.M., Phillips, B.P., Yugandhar, K., Sanford, E.J., Smolka, M.B., Yu, H., Miller, E.A., Fromme, J.C.(2021) EMBO J 40: e107607-e107607
- PubMed: 34018207
- DOI: https://doi.org/10.15252/embj.2020107607
- Primary Citation of Related Structures:
7KMT - PubMed Abstract:
The GTPase Rab1 is a master regulator of the early secretory pathway and is critical for autophagy. Rab1 activation is controlled by its guanine nucleotide exchange factor, the multisubunit TRAPPIII complex. Here, we report the 3.7 Å cryo-EM structure of the Saccharomyces cerevisiae TRAPPIII complex bound to its substrate Rab1/Ypt1. The structure reveals the binding site for the Rab1/Ypt1 hypervariable domain, leading to a model for how the complex interacts with membranes during the activation reaction. We determined that stable membrane binding by the TRAPPIII complex is required for robust activation of Rab1/Ypt1 in vitro and in vivo, and is mediated by a conserved amphipathic α-helix within the regulatory Trs85 subunit. Our results show that the Trs85 subunit serves as a membrane anchor, via its amphipathic helix, for the entire TRAPPIII complex. These findings provide a structural understanding of Rab activation on organelle and vesicle membranes.
Organizational Affiliation:
Department of Molecular Biology and Genetics/Weill Institute for Cell and Molecular Biology, Cornell University, Ithaca, NY, USA.