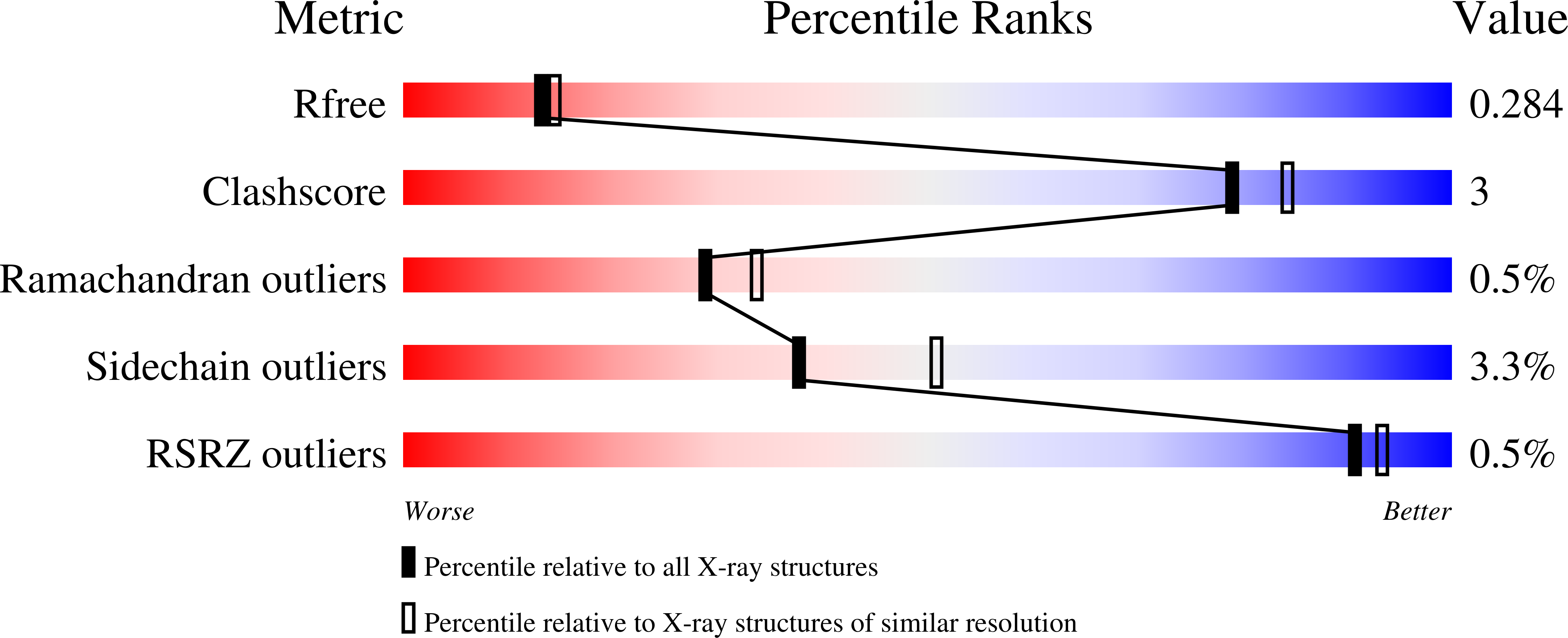
Structure-Based Discovery of Proline-Derived Arginase Inhibitors with Improved Oral Bioavailability for Immuno-Oncology.
Lu, M., Zhang, H., Li, D., Childers, M., Pu, Q., Palte, R.L., Gathiaka, S., Lyons, T.W., Palani, A., Fan, P.W., Spacciapoli, P., Miller, J.R., Cho, H., Cheng, M., Chakravarthy, K., O'Neil, J., Eangoor, P., Beard, A., Kim, H.Y., Sauri, J., Gunaydin, H., Sloman, D.L., Siliphaivanh, P., Cumming, J., Fischer, C.(2021) ACS Med Chem Lett 12: 1380-1388
- PubMed: 34527178
- DOI: https://doi.org/10.1021/acsmedchemlett.1c00195
- Primary Citation of Related Structures:
7KLK, 7KLL, 7KLM - PubMed Abstract:
Recent data suggest that the inhibition of arginase (ARG) has therapeutic potential for the treatment of a number of indications ranging from pulmonary and vascular disease to cancer. Thus, high demand exists for selective small molecule ARG inhibitors with favorable druglike properties and good oral bioavailability. In light of the significant challenges associated with the unique physicochemical properties of previously disclosed ARG inhibitors, we use structure-based drug design combined with a focused optimization strategy to discover a class of boronic acids featuring a privileged proline scaffold with superior potency and oral bioavailability. These compounds, exemplified by inhibitors 4a , 18 , and 27 , demonstrated a favorable overall profile, and 4a was well tolerated following multiple days of dosing at concentrations that exceed those required for serum arginase inhibition and concomitant arginine elevation in a syngeneic mouse carcinoma model.
Organizational Affiliation:
Discovery Chemistry, Computational and Structural Chemistry, Quantitative Biosciences, Discovery Process Chemistry, Discovery Oncology, Pharmacokinetics, Pharmacodynamics, and Drug Metabolism, and Analytical Research and Development, Merck & Co., Inc., 33 Avenue Louis Pasteur, Boston, Massachusetts 02115, United States.