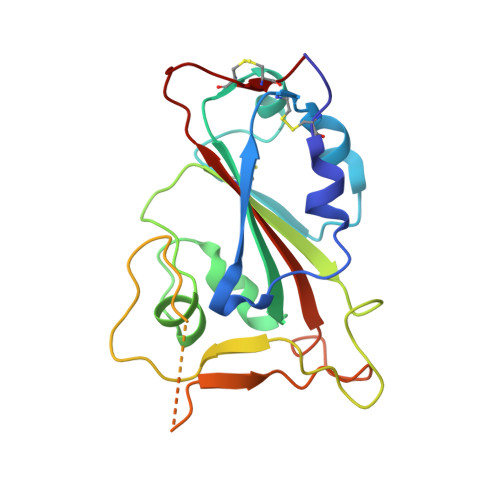
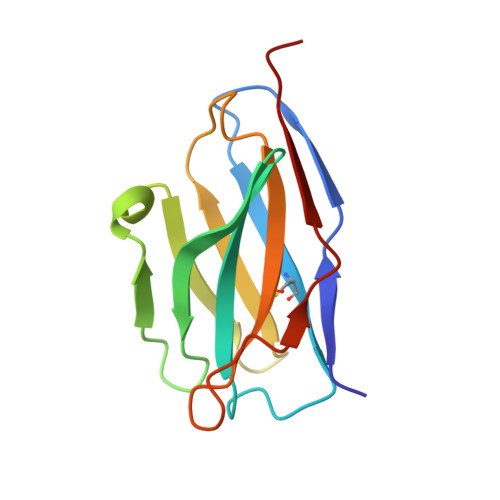
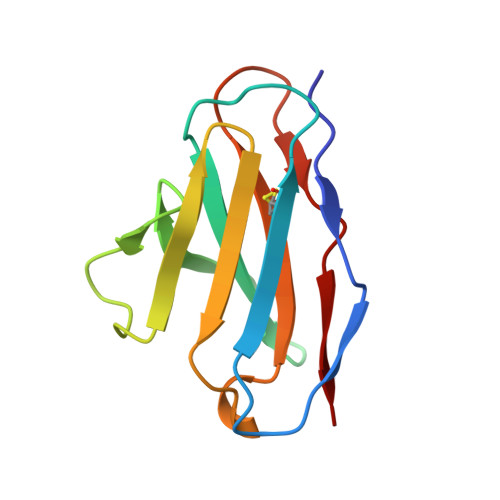
Structural mechanism of SARS-CoV-2 neutralization by two murine antibodies targeting the RBD.
Errico, J.M., Zhao, H., Chen, R.E., Liu, Z., Case, J.B., Ma, M., Schmitz, A.J., Rau, M.J., Fitzpatrick, J.A.J., Shi, P.Y., Diamond, M.S., Whelan, S.P.J., Ellebedy, A.H., Fremont, D.H.(2021) Cell Rep 37: 109881-109881
- PubMed: 34655519
- DOI: https://doi.org/10.1016/j.celrep.2021.109881
- Primary Citation of Related Structures:
7K9H, 7K9I, 7K9J, 7K9K - PubMed Abstract:
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has necessitated the rapid development of antibody-based therapies and vaccines as countermeasures. Here, we use cryoelectron microscopy (cryo-EM) to characterize two protective anti-SARS-CoV-2 murine monoclonal antibodies (mAbs) in complex with the spike protein, revealing similarities between epitopes targeted by human and murine B cells. The more neutralizing mAb, 2B04, binds the receptor-binding motif (RBM) of the receptor-binding domain (RBD) and competes with angiotensin-converting enzyme 2 (ACE2). By contrast, 2H04 binds adjacent to the RBM and does not compete for ACE2 binding. Naturally occurring sequence variants of SARS-CoV-2 and corresponding neutralization escape variants selected in vitro map to our structurally defined epitopes, suggesting that SARS-CoV-2 might evade therapeutic antibodies with a limited set of mutations, underscoring the importance of combination mAb therapeutics. Finally, we show that 2B04 neutralizes SARS-CoV-2 infection by preventing ACE2 engagement, whereas 2H04 reduces host cell attachment without directly disrupting ACE2-RBM interactions, providing distinct inhibitory mechanisms used by RBD-specific mAbs.
Organizational Affiliation:
Department of Pathology & Immunology, Washington University School of Medicine, St. Louis, MO, USA.