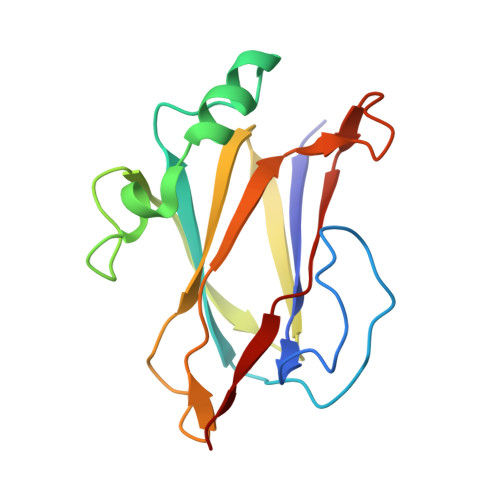
Sortase-assembled pili in Corynebacterium diphtheriae are built using a latch mechanism.
McConnell, S.A., McAllister, R.A., Amer, B.R., Mahoney, B.J., Sue, C.K., Chang, C., Ton-That, H., Clubb, R.T.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33723052
- DOI: https://doi.org/10.1073/pnas.2019649118
- Primary Citation of Related Structures:
7K7F - PubMed Abstract:
Gram-positive bacteria assemble pili (fimbriae) on their surfaces to adhere to host tissues and to promote polymicrobial interactions. These hair-like structures, although very thin (1 to 5 nm), exhibit impressive tensile strengths because their protein components (pilins) are covalently crosslinked together via lysine-isopeptide bonds by pilus-specific sortase enzymes. While atomic structures of isolated pilins have been determined, how they are joined together by sortases and how these interpilin crosslinks stabilize pilus structure are poorly understood. Using a reconstituted pilus assembly system and hybrid structural biology methods, we elucidated the solution structure and dynamics of the crosslinked interface that is repeated to build the prototypical SpaA pilus from Corynebacterium diphtheriae We show that sortase-catalyzed introduction of a K190-T494 isopeptide bond between adjacent SpaA pilins causes them to form a rigid interface in which the LPLTG sorting signal is inserted into a large binding groove. Cellular and quantitative kinetic measurements of the crosslinking reaction shed light onto the mechanism of pilus biogenesis. We propose that the pilus-specific sortase in C. diphtheriae uses a latch mechanism to select K190 on SpaA for crosslinking in which the sorting signal is partially transferred from the enzyme to a binding groove in SpaA in order to facilitate catalysis. This process is facilitated by a conserved loop in SpaA, which after crosslinking forms a stabilizing latch that covers the K190-T494 isopeptide bond. General features of the structure and sortase-catalyzed assembly mechanism of the SpaA pilus are likely conserved in Gram-positive bacteria.
Organizational Affiliation:
Department of Chemistry and Biochemistry and the University of California, Los Angeles-US Department of Energy Institute of Genomics and Proteomics, University of California, Los Angeles, CA 90095.