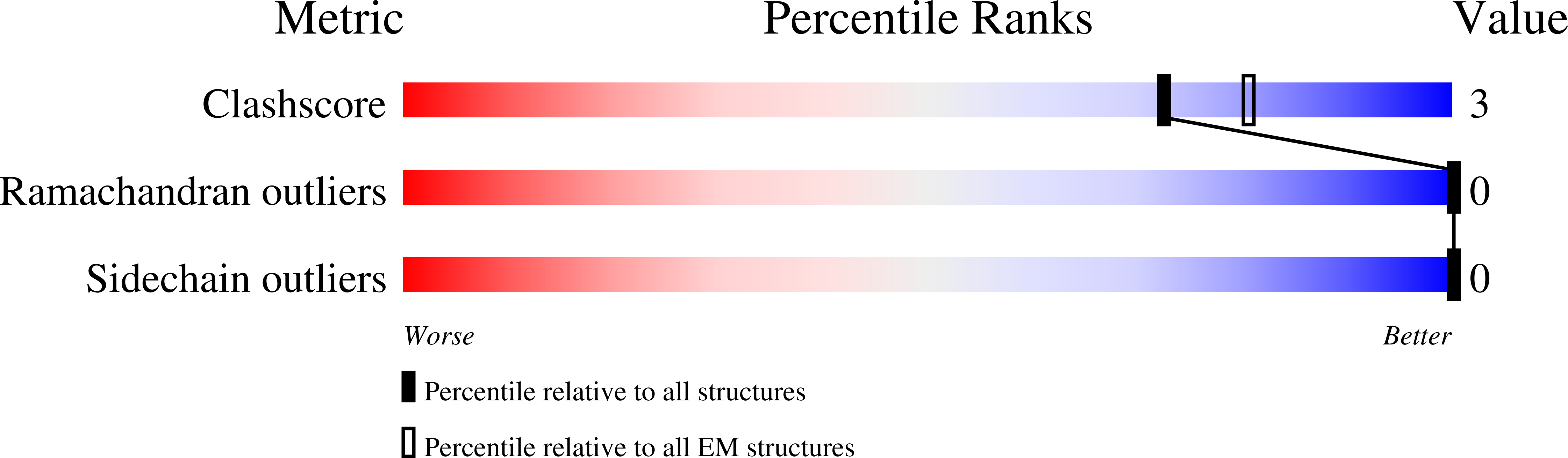Cryo-electron microscopy structures of pyrene-labeled ADP-P i - and ADP-actin filaments.
Chou, S.Z., Pollard, T.D.(2020) Nat Commun 11: 5897-5897
- PubMed: 33214556
- DOI: https://doi.org/10.1038/s41467-020-19762-1
- Primary Citation of Related Structures:
7K20, 7K21 - PubMed Abstract:
Since the fluorescent reagent N-(1-pyrene)iodoacetamide was first used to label skeletal muscle actin in 1981, the pyrene-labeled actin has become the most widely employed tool to measure the kinetics of actin polymerization and the interaction between actin and actin-binding proteins. Here we report high-resolution cryo-electron microscopy structures of actin filaments with N-1-pyrene conjugated to cysteine 374 and either ADP (3.2 Å) or ADP-phosphate (3.0 Å) in the active site. Polymerization buries pyrene in a hydrophobic cavity between subunits along the long-pitch helix with only minor differences in conformation compared with native actin filaments. These structures explain how polymerization increases the fluorescence 20-fold, how myosin and cofilin binding to filaments reduces the fluorescence, and how profilin binding to actin monomers increases the fluorescence.
Organizational Affiliation:
Department of Molecular Cellular and Developmental Biology, Yale University, PO Box 208103, New Haven, CT, 06520-8103, USA.