An allosteric inhibitor against the therapy-resistant mutant forms of EGFR in non-small cell lung cancer.
To, C., Beyett, T.S., Jang, J., Feng, W.W., Bahcall, M., Haikala, H.M., Shin, B.H., Heppner, D.E., Rana, J.K., Leeper, B.A., Soroko, K.M., Poitras, M.J., Gokhale, P.C., Kobayashi, Y., Wahid, K., Kurppa, K.J., Gero, T.W., Cameron, M.D., Ogino, A., Mushajiang, M., Xu, C., Zhang, Y., Scott, D.A., Eck, M.J., Gray, N.S., Janne, P.A.(2022) Nat Cancer 3: 402-417
- PubMed: 35422503
- DOI: https://doi.org/10.1038/s43018-022-00351-8
- Primary Citation of Related Structures:
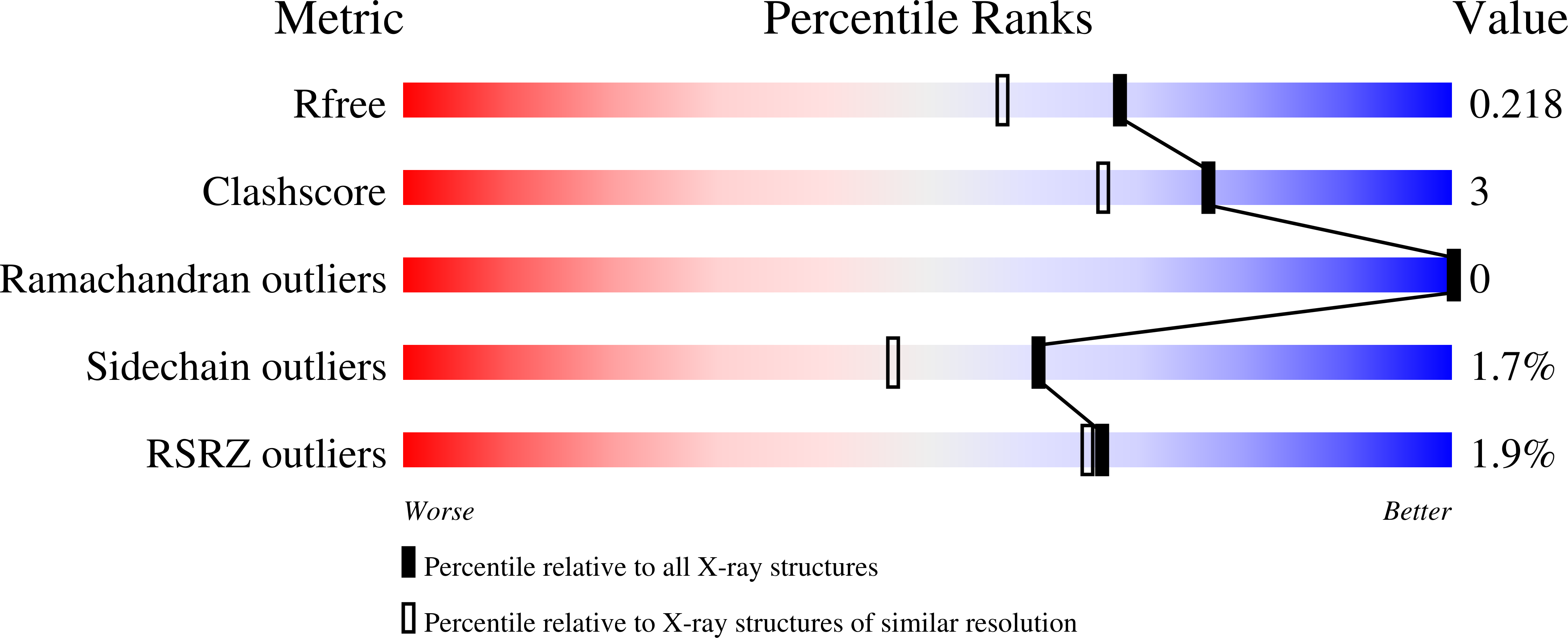
7JXQ - PubMed Abstract:
Epidermal growth factor receptor (EGFR) therapy using small-molecule tyrosine kinase inhibitors (TKIs) is initially efficacious in patients with EGFR-mutant lung cancer, although drug resistance eventually develops. Allosteric EGFR inhibitors, which bind to a different EGFR site than existing ATP-competitive EGFR TKIs, have been developed as a strategy to overcome therapy-resistant EGFR mutations. Here we identify and characterize JBJ-09-063, a mutant-selective allosteric EGFR inhibitor that is effective across EGFR TKI-sensitive and resistant models, including those with EGFR T790M and C797S mutations. We further uncover that EGFR homo- or heterodimerization with other ERBB family members, as well as the EGFR L747S mutation, confers resistance to JBJ-09-063, but not to ATP-competitive EGFR TKIs. Overall, our studies highlight the potential clinical utility of JBJ-09-063 as a single agent or in combination with EGFR TKIs to define more effective strategies to treat EGFR-mutant lung cancer.
Organizational Affiliation:
Lowe Center for Thoracic Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.