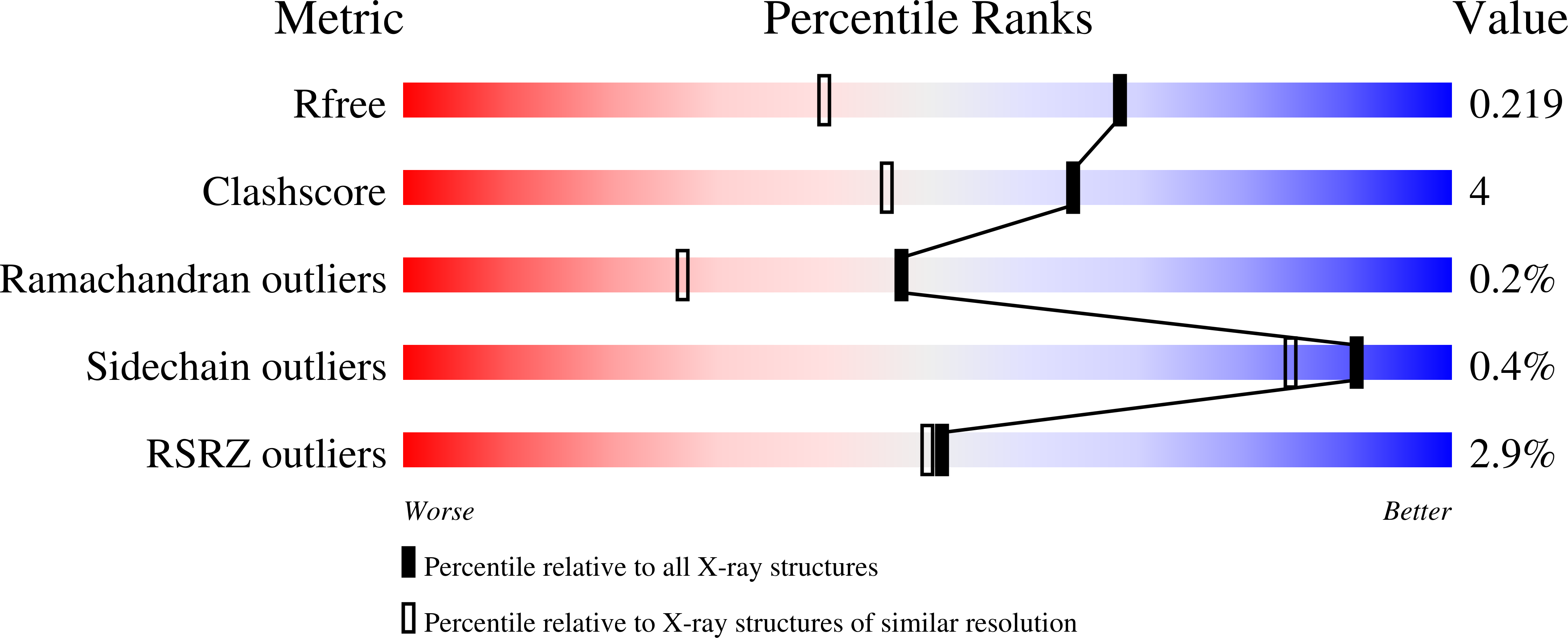
The external aldimine crystal structure of Salmonella typhimurium Tryptophan Synthase mutant beta-S377A in complex F9 inhibitor at the alpha-site and cesium ion at the metal coordination site. The single beta-Q114 rotamer conformation allows a hydrogen bond to form with the PLP oxygen at the position 3 in the ring.
Hilario, E., Dunn, M.F., Mueller, L.J.To be published.