Inhibition of Mycobacterium tuberculosis Dethiobiotin Synthase ( Mt DTBS): Toward Next-Generation Antituberculosis Agents.
Schumann, N.C., Lee, K.J., Thompson, A.P., Salaemae, W., Pederick, J.L., Avery, T., Gaiser, B.I., Hodgkinson-Bean, J., Booker, G.W., Polyak, S.W., Bruning, J.B., Wegener, K.L., Abell, A.D.(2021) ACS Chem Biol 16: 2339-2347
- PubMed: 34533923
- DOI: https://doi.org/10.1021/acschembio.1c00491
- Primary Citation of Related Structures:
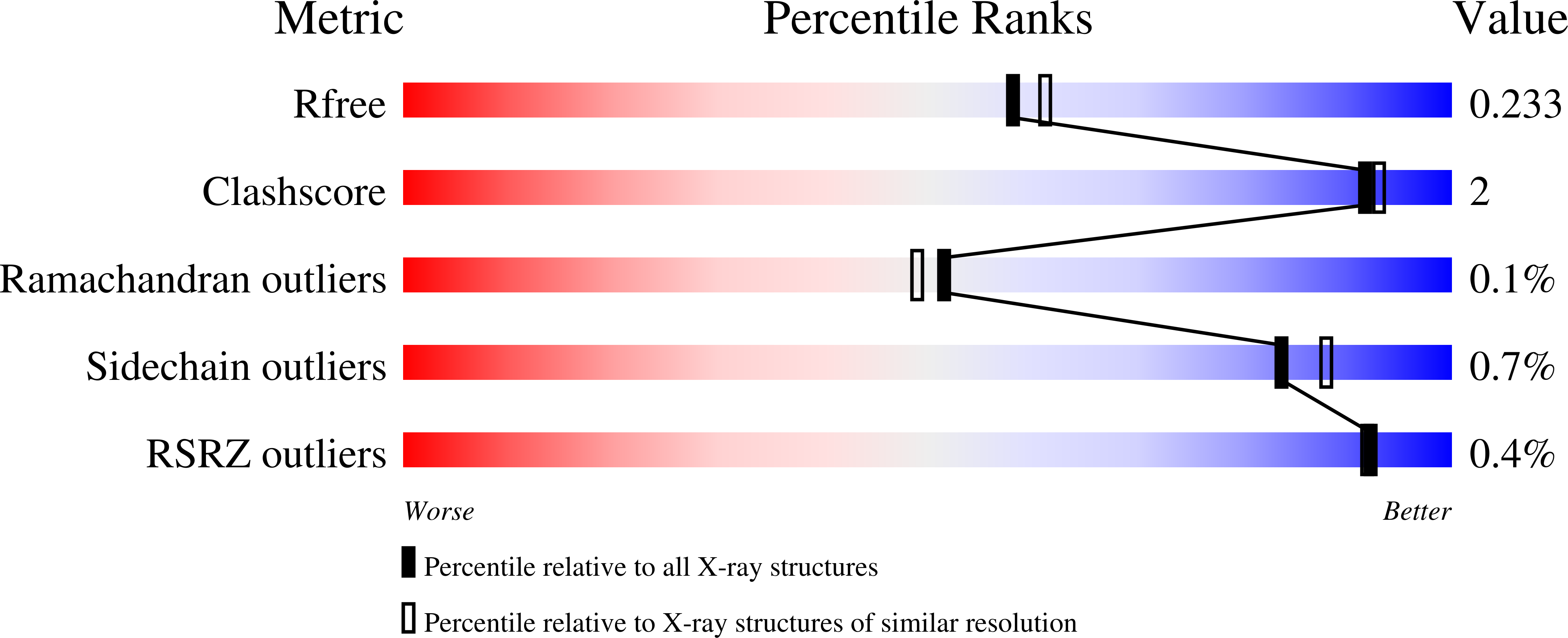
7JT5, 7JT6, 7L1J - PubMed Abstract:
Mycobacterium tuberculosis dethiobiotin synthase ( Mt DTBS) is a crucial enzyme involved in the biosynthesis of biotin in the causative agent of tuberculosis, M. tuberculosis . Here, we report a binder of Mt DTBS, cyclopentylacetic acid 2 ( K D = 3.4 ± 0.4 mM), identified via in silico screening. X-ray crystallography showed that 2 binds in the 7,8-diaminopelargonic acid (DAPA) pocket of Mt DTBS. Appending an acidic group to the para-position of the aromatic ring of the scaffold revealed compounds 4c and 4d as more potent binders, with K D = 19 ± 5 and 17 ± 1 μM, respectively. Further optimization identified tetrazole 7a as a particularly potent binder ( K D = 57 ± 5 nM) and inhibitor ( K i = 5 ± 1 μM) of Mt DTBS. Our findings highlight the first reported inhibitors of Mt DTBS and serve as a platform for the further development of potent inhibitors and novel therapeutics for the treatment of tuberculosis.
Organizational Affiliation:
Department of Chemistry, School of Physical Sciences, University of Adelaide, Adelaide, SA 5005, Australia.