Biophysical and Biochemical Characterization of TP0037, a d-Lactate Dehydrogenase, Supports an Acetogenic Energy Conservation Pathway in Treponema pallidum.
Deka, R.K., Liu, W.Z., Norgard, M.V., Brautigam, C.A.(2020) mBio 11
- PubMed: 32963009
- DOI: https://doi.org/10.1128/mBio.02249-20
- Primary Citation of Related Structures:
7JP2 - PubMed Abstract:
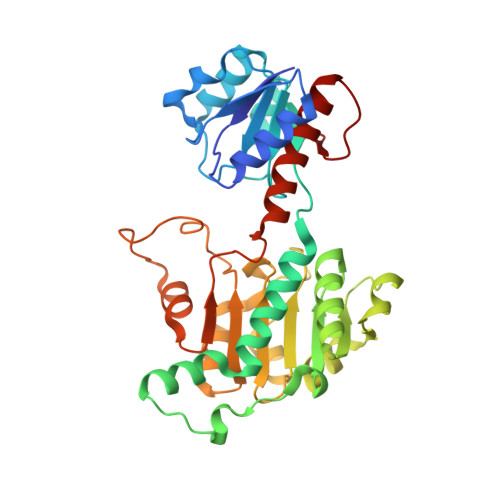
A longstanding conundrum in Treponema pallidum biology concerns how the spirochete generates sufficient energy to fulfill its complex pathogenesis processes during human syphilitic infection. For decades, it has been assumed that the bacterium relies solely on glucose catabolism (via glycolysis) for generation of its ATP. However, the organism's robust motility, believed to be essential for human tissue invasion and dissemination, would require abundant ATP likely not provided by the parsimony of glycolysis. As such, additional ATP generation, either via a chemiosmotic gradient, substrate-level phosphorylation, or both, likely exists in T. pallidum Along these lines, we have hypothesized that T. pallidum exploits an acetogenic energy conservation pathway that relies on the redox chemistry of flavins. Central to this hypothesis is the apparent existence in T. pallidum of an acetogenic pathway for the conversion of d-lactate to acetate. Herein we have characterized the structural, biophysical, and biochemical properties of the first enzyme (d-lactate dehydrogenase [d-LDH]; TP0037) predicted in this pathway. Binding and enzymatic studies showed that recombinant TP0037 consumed d-lactate and NAD + to produce pyruvate and NADH. The crystal structure of TP0037 revealed a fold similar to that of other d-acid dehydrogenases; residues in the cofactor-binding and active sites were homologous to those of other known d-LDHs. The crystal structure and solution biophysical experiments revealed the protein's propensity to dimerize, akin to other d-LDHs. This study is the first to elucidate the enzymatic properties of T. pallidum 's d-LDH, thereby providing new compelling evidence for a flavin-dependent acetogenic energy conservation (ATP-generating) pathway in T. pallidum IMPORTANCE Because T. pallidum lacks a Krebs cycle and the capability for oxidative phosphorylation, historically it has been difficult to reconcile how the syphilis spirochete generates sufficient ATP to fulfill its energy needs, particularly for its robust motility, solely from glycolysis. We have postulated the existence in T. pallidum of a flavin-dependent acetogenic energy conservation pathway that would generate additional ATP for T. pallidum bioenergetics. In the proposed acetogenic pathway, first d-lactate would be converted to pyruvate. Pyruvate would then be metabolized to acetate in three additional steps, with ATP being generated via substrate-level phosphorylation. This study provides structural, biochemical, and biophysical evidence for the first T. pallidum enzyme in the pathway (TP0037; d-lactate dehydrogenase) requisite for the conversion of d-lactate to pyruvate. The findings represent the first experimental evidence to support a role for an acetogenic energy conservation pathway that would contribute to nonglycolytic ATP production in T. pallidum .
Organizational Affiliation:
Department of Microbiology, UT Southwestern Medical Center, Dallas, Texas, USA.