Crystal structure of dopamine D1 receptor in complex with G protein and a non-catechol agonist.
Sun, B., Feng, D., Chu, M.L., Fish, I., Lovera, S., Sands, Z.A., Kelm, S., Valade, A., Wood, M., Ceska, T., Kobilka, T.S., Lebon, F., Kobilka, B.K.(2021) Nat Commun 12: 3305-3305
- PubMed: 34083522
- DOI: https://doi.org/10.1038/s41467-021-23519-9
- Primary Citation of Related Structures:
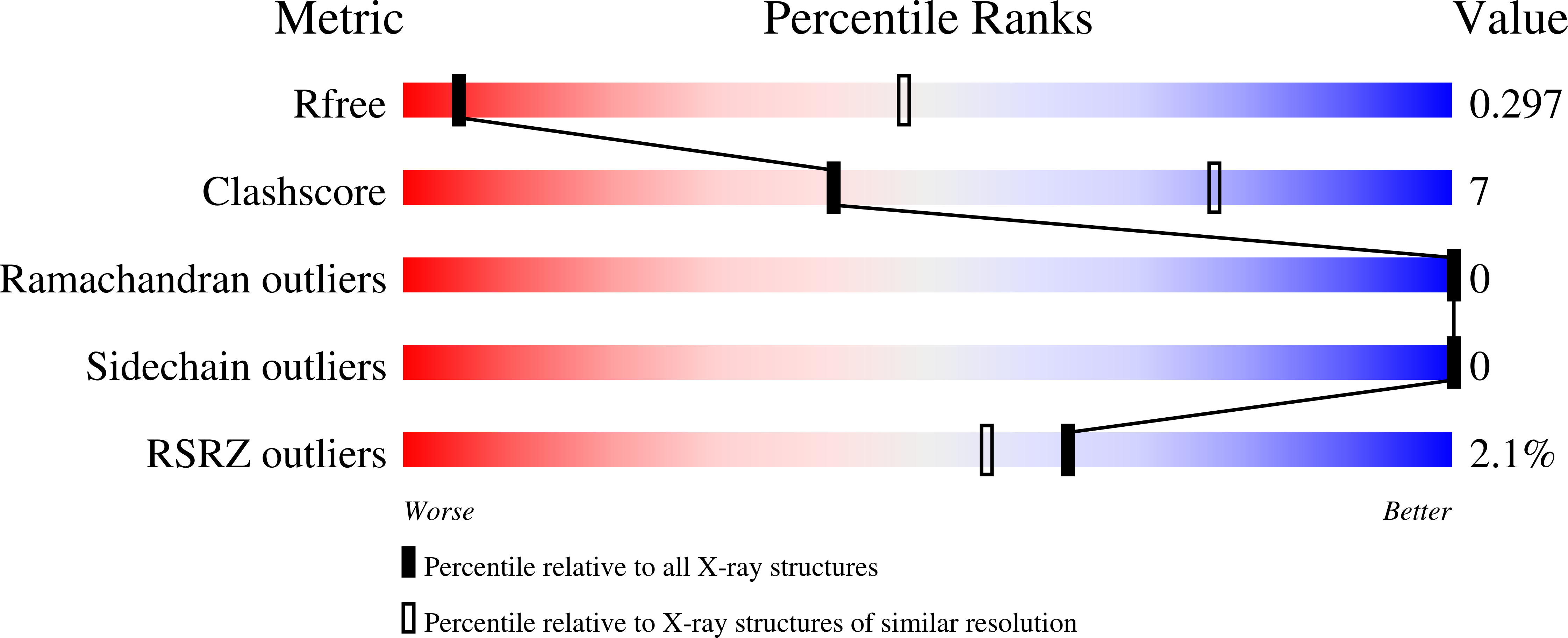
7JOZ - PubMed Abstract:
Dopamine D1 receptor (D1R) is an important drug target implicated in many psychiatric and neurological disorders. Selective agonism of D1R are sought to be the therapeutic strategy for these disorders. Most selective D1R agonists share a dopamine-like catechol moiety in their molecular structure, and their therapeutic potential is therefore limited by poor pharmacological properties in vivo. Recently, a class of non-catechol D1R selective agonists with a distinct scaffold and pharmacological properties were reported. Here, we report the crystal structure of D1R in complex with stimulatory G protein (Gs) and a non-catechol agonist Compound 1 at 3.8 Å resolution. The structure reveals the ligand bound to D1R in an extended conformation, spanning from the orthosteric site to extracellular loop 2 (ECL2). Structural analysis reveals that the unique features of D1R ligand binding pocket explains the remarkable selectivity of this scaffold for D1R over other aminergic receptors, and sheds light on the mechanism for D1R activation by the non-catechol agonist.
Organizational Affiliation:
ConfometRx, Inc., Santa Clara, CA, USA.