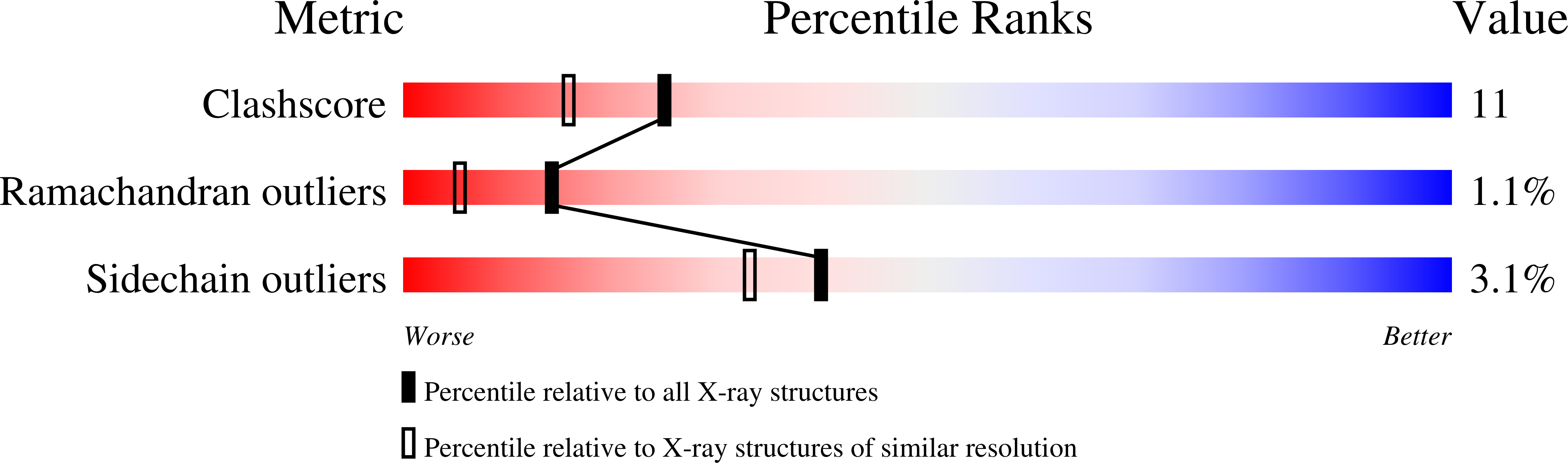Complementarity of neutron, XFEL and synchrotron crystallography for defining the structures of metalloenzymes at room temperature.
Moreno-Chicano, T., Carey, L.M., Axford, D., Beale, J.H., Doak, R.B., Duyvesteyn, H.M.E., Ebrahim, A., Henning, R.W., Monteiro, D.C.F., Myles, D.A., Owada, S., Sherrell, D.A., Straw, M.L., Srajer, V., Sugimoto, H., Tono, K., Tosha, T., Tews, I., Trebbin, M., Strange, R.W., Weiss, K.L., Worrall, J.A.R., Meilleur, F., Owen, R.L., Ghiladi, R.A., Hough, M.A.(2022) IUCrJ 9: 610-624
- PubMed: 36071813
- DOI: https://doi.org/10.1107/S2052252522006418
- Primary Citation of Related Structures:
7ADQ, 7JOR, 7KCU, 7KFM - PubMed Abstract:
Room-temperature macromolecular crystallography allows protein structures to be determined under close-to-physiological conditions, permits dynamic freedom in protein motions and enables time-resolved studies. In the case of metalloenzymes that are highly sensitive to radiation damage, such room-temperature experiments can present challenges, including increased rates of X-ray reduction of metal centres and site-specific radiation-damage artefacts, as well as in devising appropriate sample-delivery and data-collection methods. It can also be problematic to compare structures measured using different crystal sizes and light sources. In this study, structures of a multifunctional globin, dehaloperoxidase B (DHP-B), obtained using several methods of room-temperature crystallographic structure determination are described and compared. Here, data were measured from large single crystals and multiple microcrystals using neutrons, X-ray free-electron laser pulses, monochromatic synchrotron radiation and polychromatic (Laue) radiation light sources. These approaches span a range of 18 orders of magnitude in measurement time per diffraction pattern and four orders of magnitude in crystal volume. The first room-temperature neutron structures of DHP-B are also presented, allowing the explicit identification of the hydrogen positions. The neutron data proved to be complementary to the serial femtosecond crystallography data, with both methods providing structures free of the effects of X-ray radiation damage when compared with standard cryo-crystallography. Comparison of these room-temperature methods demonstrated the large differences in sample requirements, data-collection time and the potential for radiation damage between them. With regard to the structure and function of DHP-B, despite the results being partly limited by differences in the underlying structures, new information was gained on the protonation states of active-site residues which may guide future studies of DHP-B.
Organizational Affiliation:
School of Life Sciences, University of Essex, Wivenhoe Park, Colchester CO4 3SQ, United Kingdom.