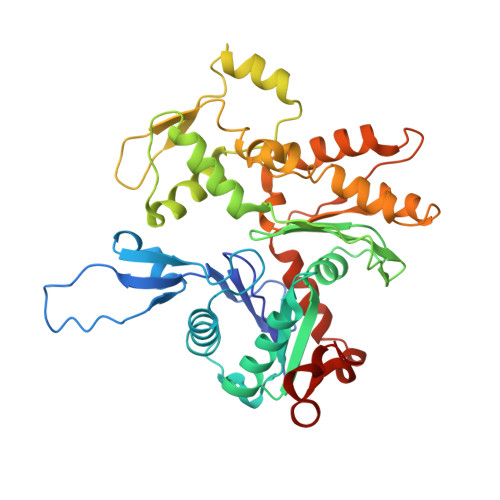
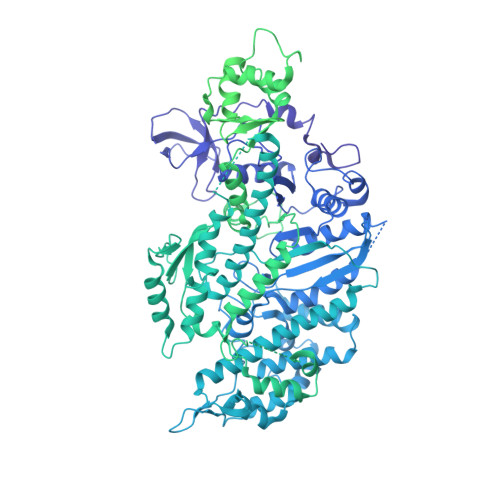
High-Resolution Cryo-EM Structure of the Cardiac Actomyosin Complex.
Risi, C., Schafer, L.U., Belknap, B., Pepper, I., White, H.D., Schroder, G.F., Galkin, V.E.(2021) Structure 29: 50
- PubMed: 33065066
- DOI: https://doi.org/10.1016/j.str.2020.09.013
- Primary Citation of Related Structures:
7JH7 - PubMed Abstract:
Heart contraction depends on a complicated array of interactions between sarcomeric proteins required to convert chemical energy into mechanical force. Cyclic interactions between actin and myosin molecules, controlled by troponin and tropomyosin, generate the sliding force between the actin-based thin and myosin-based thick filaments. Alterations in this sophisticated system due to missense mutations can lead to cardiovascular diseases. Numerous structural studies proposed pathological mechanisms of missense mutations at the myosin-myosin, actin-tropomyosin, and tropomyosin-troponin interfaces. However, despite the central role of actomyosin interactions a detailed structural description of the cardiac actomyosin interface remained unknown. Here, we report a cryo-EM structure of a cardiac actomyosin complex at 3.8 Å resolution. The structure reveals the molecular basis of cardiac diseases caused by missense mutations in myosin and actin proteins.
Organizational Affiliation:
Department of Physiological Sciences, Eastern Virginia Medical School, Norfolk, VA 23507, USA.