Structural basis for MTA1c-mediated DNA N6-adenine methylation
Chen, J., Hu, R., Chen, Y., Lin, X., Xiang, W., Chen, H., Yao, C., Liu, L.(2022) Nat Commun 13: 3257
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Transmembrane protein, putative | A [auth B], F [auth A] | 142 | Tetrahymena thermophila SB210 | Mutation(s): 0 Gene Names: p2 | 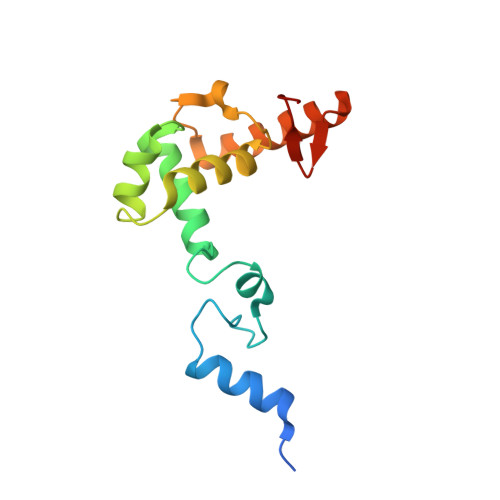 |
UniProt | |||||
Find proteins for I7M8B9 (Tetrahymena thermophila (strain SB210)) Explore I7M8B9 Go to UniProtKB: I7M8B9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | I7M8B9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MT-a70 family protein | B [auth C], C [auth D] | 247 | Tetrahymena thermophila SB210 | Mutation(s): 0 Gene Names: MTA1 | 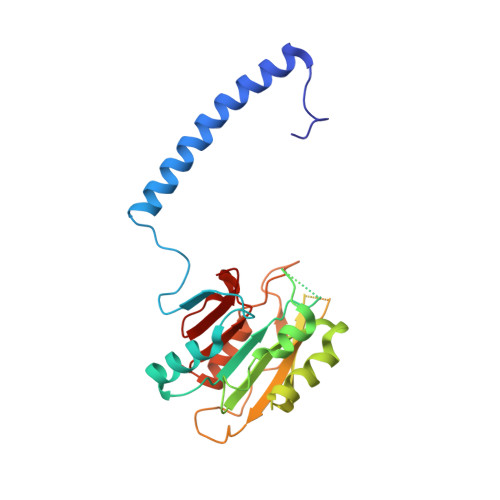 |
UniProt | |||||
Find proteins for Q22GC0 (Tetrahymena thermophila (strain SB210)) Explore Q22GC0 Go to UniProtKB: Q22GC0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q22GC0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| p1 protein | D [auth E], E [auth F] | 309 | Tetrahymena thermophila SB210 | Mutation(s): 0 | 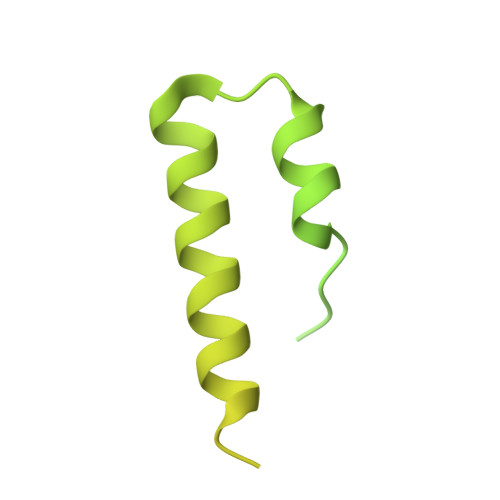 |
UniProt | |||||
Find proteins for Q22VV9 (Tetrahymena thermophila (strain SB210)) Explore Q22VV9 Go to UniProtKB: Q22VV9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q22VV9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SAM (Subject of Investigation/LOI) Query on SAM | G [auth C], H [auth D] | S-ADENOSYLMETHIONINE C15 H22 N6 O5 S MEFKEPWMEQBLKI-FCKMPRQPSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 83.517 | α = 90 |
| b = 85.786 | β = 90 |
| c = 162.903 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 32022047 |